Abstract
Background
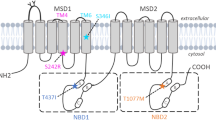
Heterozygous mutations in the gene ABCB4, encoding the phospholipid floppase MDR3 (Mdr2 in mice), are associated with various chronic liver diseases. Here we hypothesize that reduced ABCB4 expression predisposes to extrahepatic biliary atresia (EHBA).
Methods
Livers from neonatal wild-type (wt) and heterozygous Mdr2-deficient mice were subjected to mass spectrometry-based lipidomics and RNA sequencing studies. Following postnatal infection with rhesus rotavirus (RRV), liver immune responses and EHBA phenotype were assessed. Hepatic microarray data from 40 infants with EHBA were mined for expression levels of ABCB4.
Results
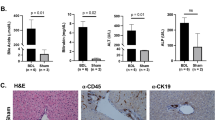
Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were increased, whereas the PC/PE ratio was decreased in neonatal Mdr2+/− mice compared with wt mice. Following RRV challenge, hepatic expression of IFNγ and infiltration with CD8+ and NK+ lymphocytes were increased in Mdr2+/− mice. Plasma total bilirubin levels and prevalence of complete ductal obstruction were higher in these mice. In infants with EHBA, hepatic gene expression of ABCB4 was downregulated in those with an inflammatory compared with a fibrosing molecular phenotype.
Conclusion
Decreased expression of ABCB4 causes dysregulation in (phospho)lipid homeostasis, and predisposes to aberrant pro-inflammatory lymphocyte responses and an aggravated phenotype of EHBA in neonatal mice. Downregulated ABCB4 is associated with an inflammatory transcriptome signature in infants with EHBA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E . The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis 2010;30:134–146.
Poupon R, Arrive L, Rosmorduc O . The cholangiographic features of severe forms of ABCB4/MDR3 deficiency-associated cholangiopathy in adults. Gastroenterol Clin Biol 2010;34:380–387.
Vij M, Safwan M, Shanmugam NP, Rela M . Liver pathology in severe multidrug resistant 3 protein deficiency: a series of 10 pediatric cases. Ann Diagn Pathol 2015;19:277–282.
Lammert F, Wang DQ, Hillebrandt S et al. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid-associated cholelithiasis. Hepatology 2004;39:117–128.
Gudbjartsson DF, Helgason H, Gudjonsson SA et al. Large-scale whole-genome sequencing of the Icelandic population. Nat Genet 2015;47:435–444.
Dixon PH, Weerasekera N, Linton KJ et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet 2000;9:1209–1217.
Ziol M, Barbu V, Rosmorduc O et al. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology 2008;135:131–141.
Degiorgio D, Crosignani A, Colombo C et al. ABCB4 mutations in adult patients with cholestatic liver disease: impact and phenotypic expression. J Gastroenterol 2015;51:271–280.
Gordo-Gilart R, Hierro L, Andueza S et al. Heterozygous ABCB4 mutations in children with cholestatic liver disease. Liver Int 2015;36:258–267.
Goldschmidt ML, Mourya R, Connor J et al. Increased frequency of double and triple heterozygous gene variants in children with intrahepatic cholestasis. Hepatol Res 2015;46:306–311.
Li Z, Agellon LB, Allen TM et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 2006;3:321–331.
Shivakumar PCK, Sabla GE, Miethke A et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329.
Li J, Bessho K, Shivakumar P et al. Th2 signals induce epithelial injury in mice and are compatible with the biliary atresia phenotype. J Clin Invest 2011;121:4244–4256.
Bessho K, Mourya R, Shivakumar P et al. Gene expression signature for biliary atresia and a role for interleukin-8 in pathogenesis of experimental disease. Hepatology 2014;60:211–223.
Moyer K, Kaimal V, Pacheco C et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med 2010;2:33.
Moustafa T, Fickert P, Magnes C et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 2012;142:e112.
Hardikar W, Ananthanarayanan M, Suchy FJ . Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem 1995;270:20841–20846.
Cui YJ, Cheng X, Weaver YM et al. Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 2009;37:203–210.
Li ZZ, Berk M, McIntyre TM et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 2009;284:5637–5644.
Devaux PF . Static and dynamic lipid asymmetry in cell membranes. Biochemistry 1991;30:1163–1173.
Walkey CJ, Yu L, Agellon LB et al. Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem 1998;273:27043–27046.
Ling J, Chaba T, Zhu LF, Jacobs RL, Vance DE . Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012;55:1094–1102.
Ikenaga N, Liu SB, Sverdlov DY et al. A new Mdr2(-/-) mouse model of sclerosing cholangitis with rapid fibrosis progression, early-onset portal hypertension, and liver cancer. Am J Pathol 2015;185:325–334.
Sontag TJ, Chellan B, Getz GS, Reardon CA . Differing rates of cholesterol absorption among inbred mouse strains yield differing levels of HDL-cholesterol. J Lipid Res 2013;54:2515–2524.
Trivedi PJ, Weston CJ, Webb GJ et al. Serum alkaline phosphatase in multidrug resistance 2 (Mdr2(-/-)) knockout mice is strain specific. Hepatology 2016;63:346.
Krones E, Erwa W, Trauner M, Fickert P . Serum alkaline phosphatase levels accurately reflect cholestasis in mice. Hepatology 2015;62:981–983.
Yan JJ, Jung JS, Lee JE et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 2004;10:161–167.
Sakata-Kaneko S, Wakatsuki Y, Usui T et al. Lysophosphatidylcholine upregulates CD40 ligand expression in newly activated human CD4+ T cells. FEBS Lett 1998;433:161–165.
Coutant F, Perrin-Cocon L, Agaugue S et al. Mature dendritic cell generation promoted by lysophosphatidylcholine. J Immunol 2002;169:1688–1695.
Asai A, Miethke A, Bezerra JA . Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 2015;12:342–352.
Shivakumar P, Sabla GE, Whitington P et al. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 2009;119:2281–2290.
Shivakumar P, Sabla G, Mohanty S et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology 2007;133:268–277.
Mezina A, Gandhi K, Sabo A et al. Abstract: whole exome sequencing identifies ABCB4 gene variants as modifiers of biliary atresia outcomes. Gastroenterology 2014;146:S-928.
Chianale J, Vollrath V, Wielandt AM et al. Fibrates induce mdr2 gene expression and biliary phospholipid secretion in the mouse. Biochem J 1996;314 (Pt 3): 781–786.
Smit JJ, Schinkel AH, Oude Elferink RP et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993;75:451–462.
Lages CS, Simmons J, Chougnet CA et al. Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 2012;56:219–227.
Lages CS, Simmons J, Maddox A et al. The dendritic cell-T helper 17-macrophage axis controls cholangiocyte injury and disease progression in murine and human biliary atresia. Hepatology 2017;65:174–188.
Miethke AG, Zhang W, Simmons J et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016;63:512–523.
Chen J, Bardes EE, Aronow BJ et al. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 2009;37:W305–W311.
Acknowledgements
We thank James Heubi (CCHMC) for the careful review of the manuscript and his thoughtful suggestions. We are grateful to Pranav Shivakumar for assistance in several of the experiments, and to Sujit Mohanty and Bryan Donnelly for providing primers and control cDNA for studies related to rotavirus replication.
Statement of financial support
The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) grant DK095001 supported this study (to A.G.M.). The project was supported in part by PHS Grant P30 DK078392 (Pathology Core, Gene expression Core, Bioinformatics) of the Digestive Disease Research Core Center in Cincinnati, by the Childhood Liver Disease Research and Education Network (ChiLDReN) Grant: 5U01DK062453-12, National Institutes of Health Level VI Fellowship Award (to A.N.C.), by the American Liver Foundation Postdoctoral Research Fellowship Award (to A.N.C.), by the 2013 American Association for the Study of Liver Diseases Fellow Research Award, by National Institutes of Health Training Grant 5T32DK007727-18 (to A.N.C.), and by the National Center for Research Resources, and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077-04.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors disclose no conflict of interest.
Rights and permissions
About this article
Cite this article
Carey, A., Zhang, W., Setchell, K. et al. Hepatic MDR3 expression impacts lipid homeostasis and susceptibility to inflammatory bile duct obstruction in neonates. Pediatr Res 82, 122–132 (2017). https://doi.org/10.1038/pr.2017.78
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.78