Abstract
Background
CD31, expressed by the majority of the neonatal T-cell pool, is involved in modulation of T-cell receptor signaling by increasing the threshold for T-cell activation. Therefore, CD31 could modulate neonatal tolerance and adaptive immune responses.
Methods
Lymphocytes were harvested from murine neonates at different ages, human late preterm and term cord blood, and adult peripheral blood. Human samples were activated over a 5-day period to simulate acute inflammation. Mice were infected with influenza; lungs and spleens were harvested at days 6 and 9 post infection and analyzed by flow cytometry.
Results
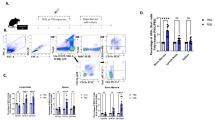
CD31-expressing neonatal murine CD4+ and CD8a+ T cells increase over the first week of life. Upon in vitro stimulation, human infants’ CD4+ and CD8a+ T cells shed CD31 faster in comparison with adults. In the context of acute infection, mice infected at 3 days of age have an increased number of naive and activated CD31+ T lymphocytes at the site of infection at days 6 and 9 post infection, as compared with those infected at 7 days of age; however, the opposite is true in the periphery.
Conclusion
Differences in trafficking of CD31+ cytotoxic T lymphocytes (CTLs) during acute influenza infection could modulate tolerance and contribute to a dampened adaptive immune response in neonates.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Martin JA, Hamilton BE, Osterman MJK Births in the United States, 2015. NCHS data brief, no 258. National Center for Health Statistics: Hyattsville, MD 2016.
Berard A, Le Tiec M, De Vera MA . Study of the costs and morbidities of late-preterm birth. Arch Dis Child Fetal Neonat Edn 2012;97:F329–F334.
Kamath AT, Rochat AF, Christensen D et al, A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS ONE 2009;4:e5771.
Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA . The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol 2010;185:2980–2988.
Gareau MG, Wine E, Reardon C, Sherman PM . Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis 2010;201:81–91.
Yasui H, Kiyoshima J, Hori T . Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol 2004;11:675–679.
Carey AJ, Gracias DT, Thayer JL et al, Rapid evolution of the CD8+ TCR repertoire in neonatal mice. J Immunol 2016;196:2602–2613.
Ma L, Mauro C, Cornish GH et al, Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc Natl Acad Sci USA 2010;107:19461–19466.
Marelli-Berg FM, Clement M, Mauro C, Caligiuri G . An immunologist's guide to CD31 function in T-cells. J Cell Sci 2013;126:2343–2352.
Privratsky JR, Newman DK, Newman PJ . PECAM-1: conflicts of interest in inflammation. Life Sci 2010;87:69–82.
Newton-Nash DK, Newman PJ . A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol 1999;163:682–688.
Scheible KM, Emo J, Yang H et al, Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin Immunol 2015;161:65–74.
Berzins SP, Boyd RL, Miller JF . The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med 1998;187:1839–1848.
Berzins SP, Godfrey DI, Miller JF, Boyd RL . A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci USA 1999;96:9787–9791.
Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G . CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology 1996;88:110–115.
Fornasa G, Groyer E, Clement M et al, TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunology 2010;184:5485–5492.
Kimmig S, Przybylski GK, Schmidt CA et al, Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med 2002;195:789–794.
Dummer W, Ernst B, LeRoy E, Lee D, Surh C . Autologous regulation of naive T cell homeostasis within the T cell compartment. J Immunol 2001;166:2460–2468.
Boyman O, Letourneau S, Krieg C, Sprent J . Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol 2009;39:2088–2094.
den Braber I, Mugwagwa T, Vrisekoop N et al, Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012;36:288–297.
Haddad R, Guimiot F, Six E et al, Dynamics of thymus-colonizing cells during human development. Immunity 2006;24:217–230.
Junge S, Kloeckener-Gruissem B, Zufferey R et al, Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol 2007;37:3270–3280.
Correa-Rocha R, Perez A, Lorente R et al, Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr Res 2012;71:590–597.
Kelly KA, Scollay R . Seeding of neonatal lymph nodes by T cells and identification of a novel population of CD3-CD4+ cells. Eur J Immunol 1992;22:329–334.
Muraro PA, Douek DC, Packer A et al, Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med 2005;201:805–816.
Kohler S, Thiel A . Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood 2009;113:769–774.
Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, de Vries E . Development of lymphocyte subpopulations in preterm infants. Scand J Immunol 2011;73:53–58.
Strunk T, Currie A, Richmond P, Simmer K, Burgner D . Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med 2011;24:25–31.
Graesser D, Solowiej A, Bruckner M et al, Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 2002;109:383–392.
Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ . Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol 2005;288:H159–H164.
Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA . Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J pathol 2005;166:185–196.
Cheung K, Ma L, Wang G et al, CD31 signals confer immune privilege to the vascular endothelium. Proc Natl Acad Sci USA 2015;112:E5815–E5824.
Scheible K, Secor-Socha S, Wightman T et al, Stability of T cell phenotype and functional assays following heparinized umbilical cord blood collection. Cytometry A 2012;81:937–949.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381.
Acknowledgements
We acknowledge the obstetrical physicians and nurses in the Labor and Delivery Unit at Hahnemann University Hospital for subject identification and sample collection.
Statement of financial support
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI108791 to AC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, funding was provided by the Margaret Q. Landenberger Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Fike, A., Nguyen, L., Kumova, O. et al. Characterization of CD31 expression on murine and human neonatal T lymphocytes during development and activation. Pediatr Res 82, 133–140 (2017). https://doi.org/10.1038/pr.2017.81
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.81