Abstract
Background
Intestinal microbiota of breast-fed infants is plenty of beneficial bifidobacteria. We aimed to determine whether an infant formula supplemented with probiotic Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM1) is effective at reducing diarrhea incidence in healthy term infants.
Methods
Double‐blinded, randomized, multicenter, controlled clinical trial, where formula-fed infants (<3 months) received an infant formula supplemented (Probiotic) or not (Control) with 107 cfu/g of B. infantis IM1 over 12 weeks. Diarrheas, growth, digestive symptoms, stool bifidobacteria, and microbiota were assessed.
Results
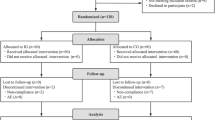
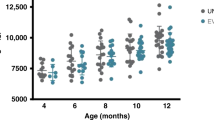
In all, 97 (Control) and 93 (Probiotic) infants were randomized, and 78 (Control) and 73 (Probiotic) completed the 12 week-follow-up. In the overall study period, a median of 0.29±1.07 and 0.05±0.28 diarrhea events/infant was observed in the Control and Probiotic groups, respectively (P=0.059). This trend to less diarrhea episodes in the Probiotic group reached statistical significance at 8 weeks (0.12±0.47 vs. 0.0±0.0 events/infant, P=0.047). Constipation incidence was higher (odds ratio (OR) 2.67 (1.09–6.50)) and stool frequency lower (2.0±1.0 vs. 2.6±1.3 stools/day, P=0.038) in the Control group after 4 weeks. No differences were found at other time points nor in other digestive symptoms, growth, or formula intake.
Conclusion
A B. infantis IM1-supplemented infant formula may reduce diarrhea episodes, being safe, well tolerated, and associated with lower constipation prevalence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Matamoros S, Gras-Leguen C, Le Vacon F et al. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 2013;21:167–73.
Gerritsen J, Smidt H, Rijkers GT et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 2011;6:209–40.
Martín R, Langa S, Reviriego C et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003;143:754–8.
Chen J, Cai W, Feng Y . Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin Nutr 2007;26:559–66.
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC et alAnalysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods.J Pediatr Gastroenterol Nutr 2007;30 (5): 61–7.
Lara-Villoslada F, Olivares M, Sierra S et al. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr 2007;98:S96–100.
Oozeer R, van Limpt K, Ludwig T et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 2013;98:561S–71S.
Saavedra JM, Abi-Hanna A, Moore N et al. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 2004;79:261–7.
Committee E, Braegger ÃC, Chmielewska ÃA et al. Supplementation of infant formula with probiotics and / or prebiotics : a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2011;52:238–50.
Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy—a randomised, double-blind, placebo-controlled study Br J Nutr 2009;101 (11): 1722–6.
Maldonado J, Cañabate F, Sempere L et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr 2012;54:55–61.
Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergic disease and food hypersensitivity Cochrane Database Syst Rev 2007 (1469-493X (Electronic)): CD006474.
Miele E, Pascarella F, Giannetti E et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 2009;104:437–443.
Guarner F, Malagelada JR . Gut flora in health and disease. Lancet 2003;361:512–9.
Mugambi MN, Musekiwa A, Lombard M et al. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J 2012;11:81.
Szajewska H, Guarino A, Hojsak I et al. Use of probiotics for management of acute gastroenteritis : a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2014;58:531–9.
Muñoz JA, Chenoll E, Casinos B et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl Environ Microbiol 2011;77:8775–83.
Carrascosa Lezcano A, Ferrández Longás A, Yeste Fernández D et al. Parte I: valores de peso y longitud en recién nacidos de 26-42 semanas de edad gestacional. An Pediatr 2008;68:544–51.
WHO WHO Anthro software for PC 2009.
Colomina J, Gil MT, Codoñer P et al. Viral proteins VP2, VP6, and NSP2 are strongly precipitated by serum and fecal antibodies from children with rotavirus symptomatic infection. J Med Virol 1998;56:58–65.
Matsuki T, Watanabe K, Fujimoto J et al. Use of 16 S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 2004;70:7220–8.
Yuan S, Cohen DB, Ravel J et al. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 2012;7:e33865.
Sim K, Cox MJ, Wopereis H et al. Improved detection of bifidobacteria with optimised 16 S rRNA-gene based pyrosequencing. PLoS ONE 2012;7:e32543.
Caporaso JG, Kuczynski J, Stombaugh J et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6.
Weizman Z, Asli G, Alsheikh A . Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005;115:5–9.
Grześkowiak Ł, Grönlund M-M, Beckmann C et al. The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe 2012;18:7–13.
Campeotto F, Suau A, Kapel N et al. A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br J Nutr 2011;105:1843–51.
Mah KW, Chin VIL, Wong WS et al. Effect of a milk formula containing probiotics on the fecal microbiota of asian infants at risk of atopic diseases. Pediatr Res 2007;62:674–9.
Di Gioia D, Aloisio I, Mazzola G et al. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol 2014;98:563–77.
Gibson RA, Barclay D, Marshall H et al. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. Br J Nutr 2009;101:1706–13.
Holscher HD, Czerkies LA, Cekola P et al. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr 2012;36 (1 Suppl): 106S–17SS.
Nagao AT, Pilagallo M, Pereira AB et al. Quantification of salivary, urinary and fecal secretory IgA, as well as in saliva titers and avidities of IgA antibodies in children living at different levels of antigenic exposure and undernutrition. Adv Exp Med Biol 1995;371A:507–11.
Weizman Z, Alsheikh A . Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr 2006;25:415–9.
Ziegler EE, Jeter JM, Drulis JM et al. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: infant growth and health. Monatsschrift Kinderheilkd 2003;151:S65–71.
Braegger C, Chmielewska A, Decsi T et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2011;52:238–50.
Chouraqui J-P, Van Egroo L-D, Fichot M-C . Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr 2004;38:288–92.
Hyams JS, Treem WR, Etienne NL et al. Effect of infant formula on stool characteristics of young infants. Pediatrics 1995;95 0031-4005 (Print) 50–4.
Vendt N, Grünberg H, Tuure T et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet 2006;19:51–8.
Tabbers MM, DiLorenzo C, Berger MY et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014;58:258–74.
Petschow BW, Figueroa R, Harris CL et al. Effects of feeding an infant formula containing Lactobacillus GG on the colonization of the intestine: a dose-response study in healthy infants. J Clin Gastroenterol 2005;39:786–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Mireia Morera, Jose Antonio Moreno Muñoz, and Montserrat Rivero are employees of ORDESA SL. Their role was to critically review the manuscript and were not involved in the statistical analysis or manuscript redaction. The remaining authors declare no conflict of interest.
Additional information
Statement of Financial Support
This clinical trial was funded by Laboratorios ORDESA SL.
Rights and permissions
About this article
Cite this article
Escribano, J., Ferré, N., Gispert-Llaurado, M. et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: a randomized controlled trial. Pediatr Res 83, 1120–1128 (2018). https://doi.org/10.1038/pr.2018.34
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2018.34
This article is cited by
-
Effectiveness and safety study of formula containing probiotics, prebiotics, synbiotics on fullterm infants’ growth - a systematic review and meta-analysis of randomized controlled study
European Journal of Clinical Nutrition (2025)
-
Bifidobacterium infantis supplementation versus placebo in early life to improve immunity in infants exposed to HIV: a protocol for a randomized trial
BMC Complementary Medicine and Therapies (2023)