Abstract
Background
Pelizaeus Merzbacher disease (PMD) is a dysmyelinating disorder of the central nervous system caused by impaired differentiation of oligodendrocytes. This study was prompted by findings that antimuscarinic compounds enhance oligodendrocyte differentiation and remyelination in vitro. One of these compounds, clemastine fumarate, is licensed for treatment of allergic conditions. We tested whether clemastine fumarate can promote myelination in two rodent PMD models, the myelin-deficient and the PLP transgenic rat.
Methods
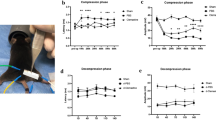
Pups were treated with daily injections of clemastine (10–30 mg/kg/day) on postnatal days 1–21. Neurologic phenotypes and myelination patterns in the brain, optic nerves, and spinal cords were assessed using histological techniques.
Results
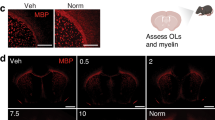
No changes in neurological phenotype or survival were observed even at the highest dose of clemastine. Postmortem staining with Luxol fast blue and myelin basic protein immunohistochemistry revealed no evidence for improved myelination in the CNS of treated rats compared to vehicle-treated littermates. Populations of mature oligodendrocytes were unaffected by the treatment.
Conclusion
These results demonstrate lack of therapeutic effect of clemastine in two rat PMD models. Both models have rapid disease progression consistent with the connatal form of the disease. Further studies are necessary to determine whether clemastine bears a therapeutic potential in milder forms of PMD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Inoue K . PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics 2005;6:1–16.
Yool DA, Edgar JM, Montague P, Malcolm S . The proteolipid protein genes and myelin disorders in man and animal models. Hum Mol Genetics 2000;9:987–992.
Nave KA, Boespflug-Tanguy O . X-linked developmental defects of myelination: from mouse mutants to human genetic disorders. Neuroscientist 1996;2:33–43.
Nave KA, Millner RJ . Proteolipid proteins: structure and genetic expression in normal and myelin-deficient mutant mice. Crit Rev Neurobiol 1989;5:65–91.
Nave K-A, Lai C, Bloom FE, Milner RJ . Jimpy mutant mouse: a 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc Natl Acad Sci USA 1986;83:9264–9268.
Nave K-A, Lai C, Bloom FE, Milner RJ . Splice site selection in the proteolipid protein (PLP) gene transcript and primary structu re of the DM-20 protein of central nervous system myelin. Proc Natl Acad Sci USA 1987;84:5665–5669.
Werner H, Jung M, Klugmann M, Sereda M, Griffiths IR, Nave KA . Mouse models of myelin diseases. Brain Pathol 1998;8:771–793.
Duncan ID, Hammang JP, Trapp BD . Abnormal compact myelin in the myelin-deficient rat: absence of proteolipid protein correlates with a defect in the intraperiod line. Proc Natl Acad Sci USA 1987;84:6287–6291.
Duncan ID, Kondo Y, Zhang S-C . The myelin mutants as models to study myelin repair in the leukodystrophies. Neurotherapeutics 2012;8:607–624.
Duncan ID, Radcliff AB . Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp Neurol 2016;283 (Pt B): 452–475.
Griffiths IR, Duncan ID, McCulloch M, Harvey JA . Shaking pups: a disorder of central myelination in the Spaniel dog. I. Clinical, genetic and light microscopical observations. J Neurol Sci 1981;50:423–433.
Mayer JA, Griffiths IR, Goldman JE et al. Modeling the natural history of Pelizaeus-Merzbacher disease. Neurobiol Dis 2015;75:115–130.
Mei F, Fancy SPJ, Shen Y-AA et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 2014;20:954–960.
Boison D, Stoffel W . Myelin-deficient rat: a point mutation in exon III (A-C, Thr-Pro) of the myelin proteolipid protein causes dysmyelination and oligodendrocyte death. EMBO J 1989;8:3295–3302.
Simons R, Riordan JR . The myelin-deficient rat has a single base substitution in the third exon of the myelin proteolipid protein gene. J Neurochem 1990;54:1079–1081.
Jackson KF, Duncan ID . Cell kinetics and cell death in the optic nerve of the myelin deficient rat. J Neurocytol 1988;17:657–670.
Grinspan JB, Coulalaglou M, Beesley JS, Carpio DF, Scherer SS . Maturation-dependent apoptotic cell death of oligodendrocytes in myelin-deficient rats. J Neurosci Res 1998;54:623–624.
Lipsitz D, Goetz BD, Duncan ID . Apoptotic glial cell death and kinetics in the spinal cord of the myelin-deficient rat. J Neurosci Res 1998;51:497–507.
Mayer JA, Larsen EC, Kondo Y, Duncan ID . Characterization of a PLP-overexpressing rat, a model for the connatal form of Pelizaeus-Merzbacher disease. Neurobiol Dis 2011;44:231–238.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier, 2007.
West MJ, Gundersen HJ . Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol 1990;296:1–22.
Xie Z, Liao Q, Li Z, Zhu C, Zeng Y, Liu S . Development and full validation of a sensitive quantitative assay for the determination of clemastine in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2007;44:924–930.
Deshmukh VA, Tardif V, Lyssiotis CA et al. A regenerative approach to the treatment of multiple sclerosis. Nature 2013;502:327–332.
Li Z, He Y, Fan S, Sun B . Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci Bull 2015;31:617–625.
Liu J, Dupree JL, Gacias M et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci 2016;36:957–962.
De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM . Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol 2012;72:713–728.
Liu J, Dietz K, DeLoyht JM et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 2012;15:1621–1623.
Voleti B, Navarria A, Liu RJ et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013;74:742–749.
Navarria A, Wohleb ES, Voleti B et al. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis 2015;82:254–261.
Wood TL, Bercury KK, Cifelli SE et al. mTOR: a link from the extracellular milieu to transcriptionalregulation of oligodendrocyte development. ASN Neuro 2013;5:e00108.
Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL . Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci 2014;34:4453–4465.
Acknowledgements
This project was supported by research funds from the Department of Neurology, University of Wisconsin, Madison
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
STATEMENT OF FINANCIAL SUPPORT
This project was supported by research funds from the Department of Neurology, University of Wisconsin, Madison
Rights and permissions
About this article
Cite this article
Turski, C., Turski, G., Chen, B. et al. Clemastine effects in rat models of a myelination disorder. Pediatr Res 83, 1200–1206 (2018). https://doi.org/10.1038/pr.2018.45
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2018.45