Abstract
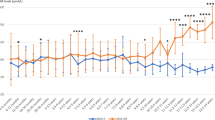
Hydroxylation of phenylalanine to tyrosine is the first and rate-limiting step in phenylalanine catabolism. Currently, there are data on the rate of phenylalanine hydroxylation in infants and adults but not in healthy children. Thus, the aim of the study reported here was to measure the rate of phenylalanine hydroxylation and oxidation in healthy school-aged children both when receiving diets with and without tyrosine. In addition, hydroxylation rates calculated from the isotopic enrichments of amino acids in plasma and in very LDL apoB-100 were compared. Eight healthy 6- to 10-y-old children were studied while receiving a control and again while receiving a tyrosine-free diet. Phenylalanine flux, hydroxylation, and oxidation were determined by a standard tracer protocol using oral administration of 13C-phenylalanine and 2H2-tyrosine for 6 h. Phenylalanine hydroxylation rate of children fed a diet devoid of tyrosine was greater than that of children fed a diet containing tyrosine (40.25 ± 5.48 versus 29.55 ± 5.35 μmol · kg−1 · h−1; p < 0.01). Phenylalanine oxidation was not different from phenylalanine hydroxylation regardless of dietary tyrosine intake, suggesting that phenylalanine converted to tyrosine was mainly oxidized. In conclusion, healthy children are capable of converting phenylalanine to tyrosine, but the need for tyrosine cannot be met by providing extra phenylalanine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- apoB:
-
apolipoprotein B
- EAR:
-
estimated average requirement
- Phe:
-
phenylalanine
- RMR:
-
resting metabolic rate
- Tyr:
-
tyrosine
References
Clarke JT, Bier DM 1982 The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of l-[ring-2H5]phenylalanine and l-[1-13C] tyrosine in the postabsorptive state. Metabolism 31: 999–1005
Cortiella J, Marchini JS, Branch S, Chapman TE, Young VR 1992 Phenylalanine and tyrosine kinetics in relation to altered protein and phenylalanine and tyrosine intakes in healthy young men. Am J Clin Nutr 56: 517–525
Thompson GN, Pacy PJ, Merritt H, Ford GC, Read MA, Cheng KN, Halliday D 1989 Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 256: E631–E639
Basile-Filho A, Beaumier L, El-Khoury A, Yu Y, Kenneway M, Gleason R, Young V 1998 Twenty-four-hour l-[1-13C]tyrosine and l-[3,3-2H2]phenylalanine oral tracer studies at generous, intermediate, and low phenylalanine intakes to estimate aromatic amino acid requirements in adults. Am J Clin Nutr 67: 640–659
Sanchez M, El-Khoury A, Castillo L, Chapman T, Filho A, Beaumier L, Young V 1996 Twenty-four-hour intravenous and oral tracer studies with l-[1-13C]phenylalanine and l-[3,3-2H2]tyrosine at a tyrosine-free, generous phenylalanine intake in adults. Am J Clin Nutr 63: 532–545
Castillo L, Yu YM, Marchini JS, Chapman TE, Sanchez M, Young VR, Burke JF 1994 Phenylalanine and tyrosine kinetics in critically ill children with sepsis. Pediatr Res 35: 580–588
Denne SC, Karn CA, Ahlrichs JA, Dorotheo AR, Wang J, Liechty EA 1996 Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest 97: 746–754
Kilani RA, Cole FS, Bier DM 1995 Phenylalanine hydroxylase activity in preterm infants: is tyrosine a conditionally essential amino acid?. Am J Clin Nutr 61: 1218–1223
Shortland GJ, Walter JH, Fleming PJ, Halliday D 1994 Phenylalanine kinetics in sick preterm neonates with respiratory distress syndrome. Pediatr Res 36: 713–718
Di Buono M, Wykes LJ, Ball RO, Pencharz PB 2001 Total sulfur amino acid requirement in young men as determined by indicator amino acid oxidation with l-[1-13C]phenylalanine. Am J Clin Nutr 74: 756–760
Elango R, Humayun MA, Ball RO, Pencharz PB 2007 Lysine requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr 86: 360–365
Kriengsinyos W, Wykes LJ, Ball RO, Pencharz PB 2002 Oral and intravenous tracer protocols of the indicator amino acid oxidation method provide the same estimate of the lysine requirement in healthy men. J Nutr 132: 2251–2257
Mager DR, Wykes LJ, Ball RO, Pencharz PB 2003 Branched-chain amino acid requirements in school-aged children determined by indicator amino acid oxidation (IAAO). J Nutr 133: 3540–3545
Riazi R, Wykes LJ, Ball RO, Pencharz PB 2003 The total branched-chain amino acid requirement in young healthy adult men determined by indicator amino acid oxidation by use of l-[1-13C]phenylalanine. J Nutr 133: 1383–1389
Turner JM, Humayun MA, Elango R, Rafii M, Langos V, Ball RO, Pencharz PB 2006 Total sulfur amino acid requirement of healthy school-age children as determined by indicator amino acid oxidation technique. Am J Clin Nutr 83: 619–623
Hsu JW, Ball RO, Pencharz PB 2007 Evidence that phenylalanine may not provide the full needs for aromatic amino acids in children. Pediatr Res 61: 361–365
House JD, Thorpe JM, Wykes LJ, Pencharz PB, Ball RO 1998 Evidence that phenylalanine hydroxylation rates are overestimated in neonatal subjects receiving total parenteral nutrition with a high phenylalanine content. Pediatr Res 43: 461–466
Roberts SA, Ball RO, Filler RM, Moore AM, Pencharz PB 1998 Phenylalanine and tyrosine metabolism in neonates receiving parenteral nutrition differing in pattern of amino acids. Pediatr Res 44: 907–914
Reeds PJ, Hachey DL, Patterson BW, Motil KJ, Klein PD 1992 VLDL apolipoprotein B-100, a potential indicator of the isotopic labeling of the hepatic protein synthetic precursor pool in humans: studies with multiple stable isotopically labeled amino acids. J Nutr 122: 457–466
Rafii M, McKenzie JM, Roberts SA, Steiner G, Ball RO, Pencharz PB 2008 In vivo regulation of phenylalanine hydroxylation to tyrosine, studied using enrichment in apoB-100. Am J Physiol Endocrinol Metab 294: E475–E479
Institute of Medicine 2002 Dietary Recommended Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Institute of Medicine, National Academy Press, Washington, DC pp 589–768
Schoeller DA, Klein PD, Watkins JB, Heim T, MacLean WC Jr 1980 13C abundances of nutrients and the effect of variations in 13C isotopic abundances of test meals formulated for 13CO2 breath tests. Am J Clin Nutr 33: 2375–2385
Bross R, Ball RO, Pencharz PB 1998 Development of a minimally invasive protocol for the determination of phenylalanine and lysine kinetics in humans during the fed state. J Nutr 128: 1913–1919
Moon JK, Vohra FA, Valerio Jimenez OS, Puyau MR, Butte NF 1995 Closed-loop control of carbon dioxide concentration and pressure improves response of room respiration calorimeters. J Nutr 125: 220–228
Caslake M, Packard C 2000 The use of ultracentrifugation for the separation of lipoproteins. Rifai N, Warnick G, Dominiczak M (eds) Handbook of Lipoprotein Testing American Association for Clinical Chemistry, Inc. Washington, DC pp 625–646
Egusa G, Brady DW, Grundy SM, Howard BV 1983 Isopropanol precipitation method for the determination of apolipoprotein B specific activity and plasma concentrations during metabolic studies of very low density lipoprotein and low density lipoprotein apolipoprotein B. J Lipid Res 24: 1261–1267
Patterson BW, Hachey DL, Cook GL, Amann JM, Klein PD 1991 Incorporation of a stable isotopically labeled amino acid into multiple human apolipoproteins. J Lipid Res 32: 1063–1072
Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR 1989 Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol 257: E426–E438
Pencharz PB, Hsu JW, Ball RO 2007 Aromatic amino acid requirements in healthy human subjects. J Nutr 137: 1576S–1578S
Marchini JS, Castillo L, Chapman TE, Vogt JA, Ajami A, Young VR 1993 Phenylalanine conversion to tyrosine: comparative determination with l-[ring-2H5]phenylalanine and l-[1-13C]phenylalanine as tracers in man. Metabolism 42: 1316–1322
Sánchez M, El-Khoury A, Castillo L, Chapman T, Young V 1995 Phenylalanine and tyrosine kinetics in young men throughout a continuous 24-h period, at a low phenylalanine intake. Am J Clin Nutr 61: 555–570
Matthews DE, Marano MA, Campbell RG 1993 Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol 264: E109–E118
Jahoor F, Burrin DG, Reeds PJ, Frazer M 1994 Measurement of plasma protein synthesis rate in infant pig: an investigation of alternative tracer approaches. Am J Physiol 267: R221–R227
Lichtenstein AH, Cohn JS, Hachey DL, Millar JS, Ordovas JM, Schaefer EJ 1990 Comparison of deuterated leucine, valine, and lysine in the measurement of human apolipoprotein A-I and B-100 kinetics. J Lipid Res 31: 1693–1701
Moss A, Schoenheimer R 1940 The conversion of phenylalanine to tyrosine in normal rats. J Biol Chem 135: 415–429
Moldawer LL, Kawamura I, Bistrian BR, Blackburn GL 1983 The contribution of phenylalanine to tyrosine metabolism in vivo. Studies in the post-absorptive and phenylalanine-loaded rat. Biochem J 210: 811–817
Shiman R, Gray DW 1998 Formation and fate of tyrosine. Intracellular partitioning of newly synthesized tyrosine in mammalian liver. J Biol Chem 273: 34760–34769
FAO/WHO/UNU 2007 Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation (World Health Organization Technical Report Series #935), Geneva, Switzerland, FAO/WHO/UNU
Ball RO, Courtney-Martin G, Pencharz PB 2006 The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J Nutr 136: 1682S–1693S
Laidlaw SA, Kopple JD 1987 Newer concepts of the indispensable amino acids. Am J Clin Nutr 46: 593–605
Pencharz PB, House JD, Wykes LJ, Ball RO 1996 What are the essential amino acids for the preterm and term infant? Bindels JB, Goedhart AC, Visser HK (eds) Recent Developments in Infant Nutrition, 10th Nutricia Symposium. Kluwer Academic Publishers, Dordrecht, Netherlands pp 278–296
Steinfeld R, Kohlschutter A, Ullrich K, Lukacs Z 2004 Efficiency of long-term tetrahydrobiopterin monotherapy in phenylketonuria. J Inherit Metab Dis 27: 449–453
Acknowledgements
We thank Cynthia Boutte for recruiting subjects and coordinating the study, Ann McMeans for designing the diet, the research nurses and kitchen staff at the MRU of the CNRC for their dedicated subject care, the staff at the Calorimeter Core Laboratory for assisting with the room respiratory calorimetry, and Rebecca Newsom for administration work. We also thank Margaret Frazer, Melanie Delrosario, Shaji Chacko, and Dan Donaldson for technical assistance. Special thanks to the subjects who participated in this study and their parents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a USDA/ARS Cooperative Agreement.This work is a publication of the US Department of Agriculture/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, Texas.The contents of this publication do not necessarily reflect the view or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Rights and permissions
About this article
Cite this article
Hsu, J., Jahoor, F., Butte, N. et al. Rate of Phenylalanine Hydroxylation in Healthy School-Aged Children. Pediatr Res 69, 341–346 (2011). https://doi.org/10.1203/PDR.0b013e31820bbdcd
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e31820bbdcd