Abstract
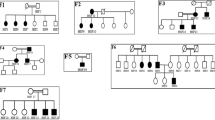
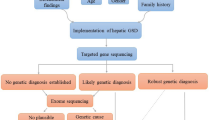
Pseudo-Bartter/Gitelman syndrome (p-BS/GS) encompasses a clinically heterogeneous group of inherited or acquired disorders similar to Bartter syndrome (BS) or Gitelman syndrome (GS), both renal salt-losing tubulopathies. Phenotypic overlap frequently occurs between p-BS/GS and BS/GS, which are difficult to diagnose based on their clinical presentation and require genetic tests for accurate diagnosis. In addition, p-BS/GS can occur as a result of other inherited diseases such as cystic fibrosis, autosomal dominant hypocalcemia, Dent disease, or congenital chloride diarrhea (CCD). However, the detection of the variants in genes other than known BS/GS-causing genes by conventional Sanger sequencing requires substantial time and resources. We studied 27 cases clinically diagnosed with BS/GS, but with negative genetic tests for known BS/GS genes. We conducted targeted sequencing for 22 genes including genes responsible for tubulopathies and other inherited diseases manifesting with p-BS/GS symptoms. We detected the SLC26A3 gene variants responsible for CCD in two patients. In Patient 1, we found the SLC26A3 compound heterozygous variants: c.354delC and c.1008insT. In Patient 2, we identified the compound heterozygous variants: c.877G > A, p.(Glu293Lys), and c.1008insT. Our results suggest that a comprehensive genetic screening system using targeted sequencing is useful for the diagnosis of patients with p-BS/GS with alternative genetic origins.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet. 1997;17:171–8.
Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet. 2001;29:310–4.
Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011;26:1789–802.
Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–8.
Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K + channel, ROMK. Nat Genet. 1996;14:152–6.
Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30.
Matsunoshita N, Nozu K, Shono A, Nozu Y, Fu XJ, Morisada N, et al. Differential diagnosis of Bartter syndrome, Gitelman syndrome, and pseudo-Bartter/Gitelman syndrome based on clinical characteristics. Genet Med. 2016;18:180–8.
Kennedy JD, Dinwiddie R, Daman-Willems C, Dillon MJ, Matthew DJ. Pseudo-Bartter’s syndrome in cystic fibrosis. Arch Dis Child. 1990;65:786–7.
Kamiyoshi N, Nozu K, Urahama Y, Matsunoshita N, Yamamura T, Minamikawa S, et al. Pathogenesis of hypokalemia in autosomal dominant hypocalcemia type 1. Clin Exp Nephrol. 2016;20:253–7.
Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, et al. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–66.
Bogdanovic R, Draaken M, Toromanovic A, Dordevic M, Stajic N, Ludwig M. A novel CLCN5 mutation in a boy with Bartter-like syndrome and partial growth hormone deficiency. Pediatr Nephrol. 2010;25:2363–8.
Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA. 2009;106:19096–101.
Antoniadi T, Buxton C, Dennis G, Forrester N, Smith D, Lunt P, et al. Application of targeted multi-gene panel testing for the diagnosis of inherited peripheral neuropathy provides a high diagnostic yield with unexpected phenotype-genotype variability. BMC Med Genet. 2015;16:84.
Lim EC, Brett M, Lai AH, Lee SP, Tan ES, Jamuar SS, et al. Next-generation sequencing using a pre-designed gene panel for the molecular diagnosis of congenital disorders in pediatric patients. Hum Genom. 2015;9:33.
Mori T, Hosomichi K, Chiga M, Mandai S, Nakaoka H, Sohara E, et al. Comprehensive genetic testing approach for major inherited kidney diseases, using next-generation sequencing with a custom panel. Clin Exp Nephrol. 2017;21:63–75.
Polla DL, Cardoso MT, Silva MC, Cardoso IC, Medina CT, Araujo R, et al. Use of targeted exome sequencing for molecular diagnosis of skeletal disorders. PLoS ONE. 2015;10:e0138314.
Ishimori S, Kaito H, Matsunoshita N, Otsubo H, Hashimoto F, Ninchoji T, et al. SLC26A3 gene analysis in patients with Bartter and Gitelman syndromes and the clinical characteristics of patients with unidentified mutations. Kobe J Med Sci. 2013;59:E36–43.
Peters M, Jeck N, Reinalter S, Leonhardt A, Tonshoff B, Klaus GG, et al. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–90.
Holmberg C, Perheentupa J, Launiala K, Hallman N. Congenital chloride diarrhoea. Clinical analysis of 21 Finnish patients. Arch Dis Child. 1977;52:255–67.
Norio R, Perheentupa J, Launiala K, Hallman N. Congenital chloride diarrhea, an autosomal recessive disease. Genetic study of 14 Finnish and 12 other families. Clin Genet. 1971;2:182–92.
Wedenoja S, Pekansaari E, Hoglund P, Makela S, Holmberg C, Kere J. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2011;32:715–22.
Lee DH, Park YK. Antenatal differential diagnosis of congenital chloride diarrhea: a case report. J Obstet Gynaecol Res. 2012;38:957–61.
Saneian H, Bahraminia E. Congenital chloride diarrhea misdiagnosed as pseudo-Bartter syndrome. J Res Med Sci. 2013;18:822–4.
Zelikovic I. Hypokalaemic salt-losing tubulopathies: an evolving story. Nephrol Dial Transplant. 2003;18:1696–1700.
Laghmani K, Beck BB, Yang SS, Seaayfan E, Wenzel A, Reusch B, et al. Polyhydramnios, transient antenatal Bartter’s syndrome, and MAGED2 mutations. N Engl J Med. 2016;374:1853–63.
Funding
This study was supported by a grant from the Ministry of Health, Labour and Welfare (Japan) for Research on Rare Intractable Diseases in the Kidney and Urinary Tract (H24-nanchitou (nan)-ippan-041 to Kazumoto Iijima) in the “Research on Measures for Intractable Diseases” Project and a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Subject ID: 15K09691 to KN and 26293203 to KI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Matsunoshita, N., Nozu, K., Yoshikane, M. et al. Congenital chloride diarrhea needs to be distinguished from Bartter and Gitelman syndrome. J Hum Genet 63, 887–892 (2018). https://doi.org/10.1038/s10038-018-0470-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-018-0470-7
This article is cited by
-
Congenital chloride diarrhoea in a Chinese infant with a compound heterozygous SLC26A3 mutation
BMC Pediatrics (2024)
-
Metabolic Alkalosis, Hypokalemia with Diarrhea due to Congenital Chloride Diarrhea
Indian Journal of Pediatrics (2024)
-
Approach to Congenital Diarrhea and Enteropathies (CODEs)
Indian Journal of Pediatrics (2024)
-
The genetics of monogenic intestinal epithelial disorders
Human Genetics (2023)
-
Monogenic mutations in four cases of neonatal-onset watery diarrhea and a mutation review in East Asia
Orphanet Journal of Rare Diseases (2021)