Abstract
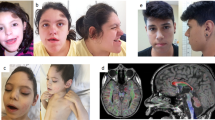
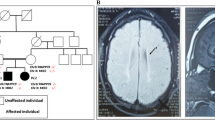
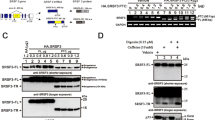
Microcephaly-capillary malformation syndrome is a congenital and neurodevelopmental disorder caused by biallelic mutations in the STAMBP gene. Here we identify the novel homozygous mutation located in the SH3 binding motif of STAMBP (NM_006463.4) (c.707C>T: p.Ser236Phe) through whole-exome sequencing. The case patient was a 2-year-old boy showing severe global developmental delay, progressive microcephaly, refractory seizures, dysmorphic facial features, and multiple capillary malformations. Immunoblot analysis of patient-derived lymphoblastoid cell lines (LCLs) revealed a severe reduction in STAMBP expression, indicating that Ser236Phe induces protein instability. STAMBP interacts with the SH3 domain of STAM and transduces downstream signals from the Jaks-STAM complex. The substitution of Ser236Phe found in the case patient was located in the SH3-binding motif, and we propose the mutation may block STAM binding and subsequently induce STAMBP degradation. Contrary to previously reported STAMBP mutations, the Ser236Phe mutation did not lead to constitutive activation of the PI3K-AKT-mTOR pathway in patient-derived LCLs, as indicated by the expression of phosphorylated S6 ribosomal protein, suggesting that it is not the major pathomechanism underlying the disorder in this patient.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
McDonell LM, Mirzaa GM, Alcantara D, Schwartzentruber J, Carter MT, Lee LJ, et al. Mutations in STAMBP, encoding a deubiquitinating enzyme, cause microcephaly-capillary malformation syndrome. Nat Genet. 2013;45:556–62.
Carter MT, Geraghty MT, De La Cruz L, Reichard RR, Boccuto L, Schwartz CE, et al. A new syndrome with multiple capillary malformations, intractable seizures, and brain and limb anomalies. Am J Med Genet A. 2011;155:301–6.
Mirzaa GM, Paciorkowski AR, Smyser CD, Willing MC, Lind AC, Dobyns WB. The microcephaly-capillary malformation syndrome. Am J Med Genet A. 2011;155:2080–7.
Isidor B, Barbarot S, Beneteau C, Le Caignec C, David A. Multiple capillary skin malformations, epilepsy, microcephaly, mental retardation, hypoplasia of the distal phalanges: report of a new case and further delineation of a new syndrome. Am J Med Genet A. 2011;155:1458–60.
Faqeih EA, Bastaki L, Rosti RO, Spencer EG, Zada AP, Saleh MA, et al. Novel STAMBP mutation and additional findings in an Arabic family. Am J Med Genet A. 2015;167:805–9.
Pavlovic M, Neubauer D, Al Tawari A, Heberle LC. The microcephaly-capillary malformation syndrome in two brothers with novel clinical features. Pediatr Neurol. 2014;51:560–5.
Naseer MI, Sogaty S, Rasool M, Chaudhary AG, Abutalib YA, Walker S, et al. Microcephaly-capillary malformation syndrome: brothers with a homozygous STAMBP mutation, uncovered by exome sequencing. Am J Med Genet A. 2016;170:3018–22.
Negishi Y, Miya F, Hattori A, Mizuno K, Hori I, Ando N, et al. Truncating mutation in NFIA causes brain malformation and urinary tract defects. Hum Genome Var. 2015;2:15007.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Sierra MI, Wright MH, Nash PD. AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4. J Biol Chem. 2010;285:13990–4004.
Tanaka N, Kaneko K, Asao H, Kasai H, Endo Y, Fujita T, et al. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J Biol Chem. 1999;274:19129–35.
Suzuki S, Tamai K, Watanabe M, Kyuuma M, Ono M, Sugamura K, et al. AMSH is required to degrade ubiquitinated proteins in the central nervous system. Biochem Biophys Res Commun. 2011;408:582–8.
Ishii N, Owada Y, Yamada M, Miura S, Murata K, Asao H, et al. Loss of neurons in the hippocampus and cerebral cortex of AMSH-deficient mice. Mol Cell Biol. 2001;21:8626–37.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45.
Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355–69.
Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Burns L, McAnally L, et al. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia. 2000;41:1540–5.
Acknowledgements
This study was supported in part by a grant for Research on Applying Health Technology from the Ministry of Health, Labour and Welfare of Japan to F.M., N.O., M.K., M.Y., Y.K., K.K., and SS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hori, I., Miya, F., Negishi, Y. et al. A novel homozygous missense mutation in the SH3-binding motif of STAMBP causing microcephaly-capillary malformation syndrome. J Hum Genet 63, 957–963 (2018). https://doi.org/10.1038/s10038-018-0482-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-018-0482-3
This article is cited by
-
STAMBP is Required for Long-Term Maintenance of Neural Progenitor Cells Derived from hESCs
Stem Cell Reviews and Reports (2024)