Abstract
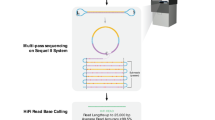
The recent advent of long-read sequencing technologies is expected to provide reasonable answers to genetic challenges unresolvable by short-read sequencing, primarily the inability to accurately study structural variations, copy number variations, and homologous repeats in complex parts of the genome. However, long-read sequencing comes along with higher rates of random short deletions and insertions, and single nucleotide errors. The relatively higher sequencing accuracy of short-read sequencing has kept it as the first choice of screening for single nucleotide variants and short deletions and insertions. Albeit, short-read sequencing still suffers from systematic errors that tend to occur at specific positions where a high depth of reads is not always capable to correct for these errors. In this study, we compared the genotyping of mitochondrial DNA variants in three samples using PacBio’s Sequel (Pacific Biosciences Inc., Menlo Park, CA, USA) long-read sequencing and illumina’s HiSeqX10 (illumine Inc., San Diego, CA, USA) short-read sequencing data. We concluded that, despite the differences in the type and frequency of errors in the long-reads sequencing, its accuracy is still comparable to that of short-reads for genotyping short nuclear variants; due to the randomness of errors in long reads, a lower coverage, around 37 reads, can be sufficient to correct for these random errors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chaisson MJP, Huddleston J, Dennis MY, Sudmant PH, Malig M, Hormozdiari F, et al. Resolving the complexity of the human genome using singlemolecule sequencing. Nature. 2015;517:608–U163.
Pollard MO, Gurdasani D, Mentzer AJ, Porter T, Sandhu MS. Long reads: their purpose and place. Hum Mol Genet. 2018;27(R2):R234–41.
Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–8.
Jain M, Koren S, Miga KH, Quick J, Rand AC, Sasani TA, et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat Biotechnol. 2018;36:338.
Weirather JL, de Cesare M, Wang Y, Piazza P, Sebastiano V, Wang XJ, et al. Comprehensive comparison of Pacific Biosciences and Oxford Nanopore Technologies and their applications to transcriptome analysis. F1000Res. 2017;6:100.
Hestand MS, Van Houdt J, Cristofoli F, Vermeesch JR. Polymerase specific error rates and profiles identified by single molecule sequencing. Mutat Res-Fundam Mol Mech Mutagen. 2016;784:39–45.
Potapov V, Ong JL. Examining sources of error in PCR by Single-molecule sequencing. PLoS ONE. 2017;12:e0169774.
Guiblet WM, Cremona MA, Cechova M, Harris RS, Kejnovská I, Kejnovsky E, et al. Long-read sequencing technology indicates genome-wide effects of non-B DNA on polymerization speed and error rate. Genome Res. 2018;28:1767–78.
Wenger AM, Peluso P, Rowell WJ, Chang P-C, Hall RJ, Concepcion GT, et al. Highly-accurate long-read sequencing improves variant detection and assembly of a human genome. bioRxiv, 2019:519025.
Vollger MR, Logsdon GA, Audano PA, Sulovari A, Porubsky D, Peluso P, et al. Improved assembly and variant detection of a haploid human genome using single-molecule, high-fidelity long reads. bioRxiv, 2019:635037.
Nakamura K, Oshima T, Morimoto T, Ikeda S, Yoshikawa H, Shiwa Y, et al. Sequence-specific error profile of Illumina sequencers. Nucleic Acids Res. 2011;39:e90.
Pfeiffer F, Gröber C, Blank M, Händler K, Beyer M, Schultze JL, et al. Systematic evaluation of error rates and causes in short samples in next-generation sequencing. Sci Rep. 2018;8:10950.
Guo XG, Lehner K, O’Connell K, Zhang J, Dave SS, Jinks-Robertson S. SMRT sequencing for parallel analysis of multiple targets and accurate SNP phasing. G3-genes genomes. Genetics. 2015;5:2801–8.
Ebler J, Haukness M, Pesout T, Marschall T, Paten B. Haplotype-aware genotyping from noisy long reads. Genome Biol. 2019;20:116.
Erik Garrison GM Haplotype-based variant detection from short-read sequencing. arXiv. 2012;arXiv:1207.3907.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–34.
Chaisson MJP, Sanders AD, Zhao X, Malhotra A, Porubsky D, Rausch T, et al. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat Commun. 2019;10:1784.
Mizuguchi T, Toyota T, Adachi H, Miyake N, Matsumoto N, Miyatake S. Detecting a long insertion variant in SAMD12 by SMRT sequencing: implications of long-read whole-genome sequencing for repeat expansion diseases. J Hum Genet. 2019;64:191–7.
Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66–7.
Chaisson MJ, Tesler G. Mapping single molecule sequencing reads using basic local alignment with successive refinement (BLASR): application and theory. Bmc Bioinformatics. 2012;13:238.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–9.
Behar DM, van Oven M, Rosset S, Metspalu M, Loogväli EL, Silva NM, et al. A “Copernican” Reassessment of the Human Mitochondrial DNA Tree from its Root. Am J Hum Genet. 2012;90:675–84.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–9.
Kloss-Brandstatter A, Weissensteiner H, Erhart G, Schäfer G, Forer L, Schönherr S, et al. Validation of next-generation sequencing of entire mitochondrial genomes and the diversity of mitochondrial DNA Mutations in oral squamous cell carcinoma. PLoS ONE. 2015;10:e0135643.
Weissensteiner H, Forer L, Fuchsberger C, Schöpf B, Kloss-Brandstätter A, Specht G, et al. mtDNA-Server: next-generation sequencing data analysis of human mitochondrial DNA in the cloud. Nucleic Acids Res. 2016;44(W1):W64–9.
Li MK, Schönberg A, Schaefer M, Schroeder R, Nasidze I, Stoneking M. Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am J Hum Genet. 2010;87:237–49.
Zhidkov I, Nagar T, Mishmar D, Rubin E. MitoBamAnnotator: a web-based tool for detecting and annotating heteroplasmy in human mitochondrial DNA sequences. Mitochondrion. 2011;11:924–8.
Spencer DH, Tyagi M, Vallania F, Bredemeyer AJ, Pfeifer JD, Mitra RD, et al. Performance of common analysis methods for detecting low-frequency single nucleotide variants in targeted next-generation sequence data. J Mol Diagn. 2014;16:75–88.
Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46.
Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–82.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Beck TF, Mullikin JC. NISC Comparative Sequencing Program, Biesecker LG. Systematic evaluation of sanger validation of next-generation sequencing variants. Clin Chem. 2016;62:647–54.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45.
Acknowledgements
This work was supported by AMED under the grant numbers JP19ek0109280, JP19dm0107090, JP19ek0109301, JP19ek0109348, and JP18kk020501; JSPS KAKENHI grant numbers JP17H01539, JP16H05357, JP16H06254, JP17K10080, and JP 17K15630; the Takeda Science Foundation; and the Ichiro Kanehara Foundation for the Promotion of Medical Science and Medical Care. We also thank T. Miyama for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alkanaq, A.N., Hamanaka, K., Sekiguchi, F. et al. Comparison of mitochondrial DNA variants detection using short- and long-read sequencing. J Hum Genet 64, 1107–1116 (2019). https://doi.org/10.1038/s10038-019-0654-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-019-0654-9
This article is cited by
-
Targeted nanopore sequencing using the Flongle device to identify mitochondrial DNA variants
Scientific Reports (2024)
-
Genetic testing for mitochondrial disease: the United Kingdom best practice guidelines
European Journal of Human Genetics (2023)
-
A method for multiplexed full-length single-molecule sequencing of the human mitochondrial genome
Nature Communications (2022)
-
Applying genomic and transcriptomic advances to mitochondrial medicine
Nature Reviews Neurology (2021)