Abstract
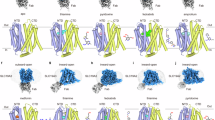
Thiamine metabolism dysfunction syndrome-4 (THMD4) includes episodic encephalopathy, often associated with a febrile illness, causing transient neurologic dysfunction and a slowly progressive axonal polyneuropathy. Until now only two mutations (G125S and S194P) have been reported in the SLC25A19 gene as causative for this disease and a third mutation (G177A) as related to the Amish lethal microcephaly. In this work, we describe the clinical and molecular features of a patient carrying a novel mutation (c.576G>C; Q192H) on SLC25A19 gene. Functional studies on this mutation were performed explaining the pathogenetic role of c.576G>C in affecting the translational efficiency and/or stability of hMTPPT protein instead of the mRNA expression. These findings support the pathogenetic role of Q192H (c.576G>C) mutation on SLC25A19 gene. Moreover, despite in other patients the thiamine supplementation leaded to a substantial improvement of peripheral neuropathy, our patient did not show a clinical improvement.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, et al. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem. 1995;64:2013–21.
Ortigoza-Escobar JD, Alfadhel M, Molero-Luis M, Darin N, Spiegel R, de Coo IF, et al. Thiamine deficiency in childhood with attention to genetic causes: survival and outcome predictors. Ann Neurol. 2017;82:317–30.
Spiegel R, Shaag A, Edvardson S, Mandel H, Stepensky P, Shalev SA, et al. SLC25A19 mutation as a cause of neuropathy and bilateral striatal necrosis. Ann Neurol. 2009;66:419–24.
Rosenberg MJ, Agarwala R, Bouffard G, Davis J, Fiermonte G, Hilliard MS, et al. Mutant deoxynucleotide carrier is associated with congenital microcephaly. Nat Genet. 2002;32:175–9.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Bottega R, Nicchia E, Cappelli E, Ravera S, De Rocco D, Faleschini M, et al. Hypomorphic FANCA mutations correlate with mild mitochondrial and clinical phenotype in Fanconi anemia. Haematologica. 2018;103:417–26.
Sabui S, Subramanian VS, Kapadia R, Said HM. Structure-function characterization of the human mitochondrial thiamin pyrophosphate transporter (hMTPPT; SLC25A19): important roles for Ile(33), Ser(34), Asp(37), His(137) and Lys(291). Biochim Biophys Acta. 2016;1858:1883–90.
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44.
Bawono P, Heringa J. PRALINE: a versatile multiple sequence alignment toolkit. Methods Mol Biol. 2014;1079:245–62.
Ravera S, Dufour C, Cesaro S, Bottega R, Faleschini M, Cuccarolo P, et al. Evaluation of energy metabolism and calcium homeostasis in cells affected by Shwachman-Diamond syndrome. Sci Rep. 2016;6:25441.
Subramanian VS, Nabokina SM, Lin-Moshier Y, Marchant JS, Said HM. Mitochondrial uptake of thiamin pyrophosphate: physiological and cell biological aspects. PLoS ONE. 2013;8:e73503.
Subramanian VS, Subramanya SB, Said HM. Relative contribution of THTR-1 and THTR-2 in thiamin uptake by pancreatic acinar cells: studies utilizing Slc19a2 and Slc19a3 knockout mouse models. Am J Physiol Gastrointest Liver Physiol. 2012;302:G572–8.
De Rocco D, Melazzini F, Marconi C, Pecci A, Bottega R, Gnan C, et al. Mutations of RUNX1 in families with inherited thrombocytopenia. Am J Hematol. 2017;92:E86–8.
Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, et al. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci USA. 2006;103:15927–32.
Foulon V, Antonenkov VD, Croes K, Waelkens E, Mannaerts GP, Van Veldhoven PP, et al. Purification, molecular cloning, and expression of 2-hydroxyphytanoyl-CoA lyase, a peroxisomal thiamine pyrophosphate-dependent enzyme that catalyzes the carbon-carbon bond cleavage during alpha-oxidation of 3-methyl-branched fatty acids. Proc Natl Acad Sci USA. 2009;96:10039–44.
Acknowledgements
This study was supported by IRCCS Burlo Garofolo (Ricerca Corrente 31/17). The authors thank the family for their participation in this project.
Author information
Authors and Affiliations
Contributions
RB, MDP, HMS, and FF designed the study, and drafted the manuscript. SS and RB performed functional studies under HMS and FF supervision. VP performed SNP-array analysis. MDP, KV, AT, and FF collected the samples and performed clinical analysis. All authors revised critically the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bottega, R., Perrone, M.D., Vecchiato, K. et al. Functional analysis of the third identified SLC25A19 mutation causative for the thiamine metabolism dysfunction syndrome 4. J Hum Genet 64, 1075–1081 (2019). https://doi.org/10.1038/s10038-019-0666-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-019-0666-5
This article is cited by
-
Pathogenic morphological signatures of perturbations in mitochondrial-related genes revealed by pooled imaging assay
npj Imaging (2025)
-
Identification and functional analysis of novel SLC25A19 variants causing thiamine metabolism dysfunction syndrome 4
Orphanet Journal of Rare Diseases (2021)