Abstract
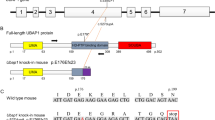
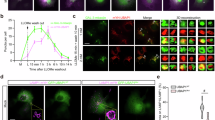
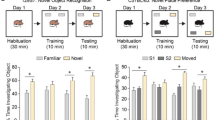
We aimed to find a new causative gene and elucidate the molecular mechanisms underlying a new type of hereditary spastic paraplegia (HSP). Patients with HSP were recruited from the Japan Spastic Paraplegia Research Consortium (JASPAC). Exome sequencing of genomic DNA from patients in four families was carried out, followed by Sanger sequencing of the UBAP1 gene. A mouse homolog of one UBAP1 frameshift mutation carried by one of the patients was created as a disease model. Functional properties of the UBAP1 wild type and UBAP1-mutant in mouse hippocampus neurons were examined. We identified three novel heterozygous loss of function mutations (c.425_426delAG, c.312delC, and c.535G>T) in the UBAP1 gene as the genetic cause of a new type of HSP (SPG80). All the patients presented identical clinical features of a pure type of juvenile-onset HSP. Functional studies on mouse hippocampal neurons revealed that the C-terminal deletion UBAP1-mutant of our disease model had lost its ability to bind ubiquitin in vitro. Overexpression of the UBAP1 wild type interacts directly with ubiquitin on enlarged endosomes, while the UBAP1-mutant cannot be recruited to endosome membranes. Our study demonstrated that mutations in the UBAP1 gene cause a new type of HSP and elucidated its pathogenesis. The full-length UBAP1 protein is involved in endosomal dynamics in neurons, while loss of UBAP1 function may perturb endosomal fusion and sorting of ubiquitinated cargos. These effects could be more prominent in neurons, thereby giving rise to the phenotype of a neurodegenerative disease such as HSP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–5.
Tesson C, Koht J, Stevanin G. Delving into the complexity of hereditary spastic paraplegias: how unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum Genet. 2015;134:511–38.
Finsterer J, Löscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;318:1–18.
Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174–83.
Klebe S, Stevanin G. Clinical and genetic heterogeneity in hereditary spastic paraplegias: from SPG1 to SPG72 and still counting. Rev Neurol. 2015;171:505–30.
Blackstone C. Hereditary spastic paraplegia. Handb Clin Neurol. 2018;148:633–52.
Schüle R, Wiethoff S, Martus P, Karle KN, Otto S, Klebe S, et al. Hereditary spastic paraplegia: clinicogenetic lessons from 608 patients. Ann Neurol. 2016;79:646–58.
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91.
Wunderley L, Brownhill K, Stefani F, Tabernero L, Woodman P. The molecular basis for selective assembly of the UBAP1-containing endosome-specific ESCRT-I complex. J Cell Sci. 2014;127:663–72.
Agromayor M, Soler N, Caballe A, Kueck T, Freund SM, Allen MD, et al. The UBAP1 subunit of ESCRT-I interacts with ubiquitin via a SOUBA domain. Structure. 2012;20:414–28.
Qian J, Yang J, Zhang X, Zhang B, Wang J, Zhou M, et al. Isolation and characterization of a novel cDNA, UBAP1, derived from the tumor suppressor locus in human chromosome 9p21-22. J Cancer Res Clin Oncol. 2001;127:613–8.
Rollinson S, Rizzu P, Sikkink S, Baker M, Halliwell N, Snowden J, et al. Ubiquitin associated protein 1 is a risk factor for frontotemporal lobar degeneration. Neurobiol Aging. 2009;30:656–65.
Koh K, Ishiura H, Tsuji S, Takiyama Y. JASPAC: Japan Spastic Paraplegia Research Consortium. Brain Sci. 2018;8:153.
Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, et al. CAST: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J Cell Biol. 2002;158:577–90.
Stefani F, Zhang L, Taylor S, Donovan J, Rollinson S, Doyotte A, et al. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr Biol. 2011;21:1245–50.
Gahloth D, Levy C, Heaven G, Stefani F, Wunderley L, Mould P, et al. Structural basis for selective interaction between the ESCRT regulator HD-PTP and UBAP1. Structure. 2016;24:2115–26.
Ali N, Zhang L, Taylor S, Mironov A, Urbé S, Woodman P. Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr Biol. 2013;23:453–61.
Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–8.
Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–86.
Shields SB, Piper RC. How ubiquitin functions with ESCRTs. Traffic. 2011;12:1306–17.
Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–23.
Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52.
Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab 5 controls early endosome fusion in vitro. Cell. 1991;64:915–25.
Numrich J, Ungermann C. Endocytic Rabs in membrane trafficking and signaling. Biol Chem. 2014;395:327–33.
Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;10:721–30.
Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;3:195–201.
Dupré S, Volland C, Haguenauer-Tsapis R. Membrane transport: ubiquitylation in endosomal sorting. Curr Biol. 2001;11:R932–R934.
Blackstone C. Cellular pathways of hereditary spastic paraplegia. Annu Rev Neurosci. 2012;35:25–47.
Yang D, Rismanchi N, Renvoisé B, Lippincott-Schwartz J, Blackstone C, Hurley JH. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat Struct Mol Biol. 2008;15:1278–86.
Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56.
Guizetti J, Schermelleh L, Mäntler J, Maar S, Poser I, Leonhardt H, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–20.
Renvoise´ B, Parker RL, Yang D, Bakowska JC, Hurley JH, Blackstone C. SPG20 protein spartin is recruited to midbodies by ESCRT-III protein Ist1 and participates in cytokinesis. Mol Biol Cell. 2010;21:3293–303.
Zivony-Elboum Y, Westbroek W, Kfir N, Savitzki D, Shoval Y, Bloom A, et al. A founder mutation in Vps37A causes autosomal recessive complex hereditary spastic paraparesis. J Med Genet. 2012;49:462–72.
Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–6.
Walker WP, Oehler A, Edinger AL, Wagner KU, Gunn TM. Oligodendroglial deletion of ESCRT-I component TSG101 causes spongiform encephalopathy. Biol Cell. 2016;108:324–37.
Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–28.
Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci USA. 2006;103:9595–600.
Deng H-X, Zhai H, Fu R, Shi Y, Gorrie GH, Yang Y, et al. Distal axonopathy in an alsin-deficient mouse model. Hum Mol Genet. 2007;16:2911–2020.
Hadano S, Kunita R, Otomo A, Suzuki-Utsunomiya K, Ikeda JE. Molecular and cellular function of ALS2/alsin: implication of membrane dynamics in neuronal development and degeneration. Neurochem Int. 2007;51:74–84.
Otomo A, Hadano S, Okada T, Mizumura H, Kunita R, Nishijima H, et al. ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum Mol Genet. 2003;12:1671–87.
Mohammad A, Adriana R, Elena B, Hamid N, Hassan D, Ina G, et al. Truncating mutations in UBAP1 cause hereditary spastic paraplegia. Am J Hum Genet. 2019;104:767–73.
Acknowledgements
We thank all the participants and cooperating doctors in the JASPAC. This work was supported by Grants-in-Aid from the Research Committee for Ataxic Disease (YT), the Ministry of Health, Labor and Welfare, Japan, JSPS KAKENHI Grant Number JP18K07495 (YT) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and grants for AMED under Grant Number JP18kk0205001h003 (YT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nan, H., Ichinose, Y., Tanaka, M. et al. UBAP1 mutations cause juvenile-onset hereditary spastic paraplegias (SPG80) and impair UBAP1 targeting to endosomes. J Hum Genet 64, 1055–1065 (2019). https://doi.org/10.1038/s10038-019-0670-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-019-0670-9
This article is cited by
-
DNA methylation signature of a lifestyle-based resilience index for cognitive health
Alzheimer's Research & Therapy (2025)
-
Hereditäre spastische Spinalparalysen erkennen und behandeln
NeuroTransmitter (2024)
-
Ubap1 knock-in mice reproduced the phenotype of SPG80
Journal of Human Genetics (2022)
-
Challenges and Controversies in the Genetic Diagnosis of Hereditary Spastic Paraplegia
Current Neurology and Neuroscience Reports (2021)
-
Identification of UBAP1 mutations in juvenile hereditary spastic paraplegia in the 100,000 Genomes Project
European Journal of Human Genetics (2020)