Abstract
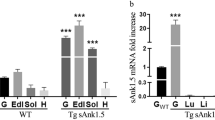
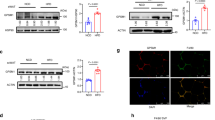
Genome-wide association studies (GWASs) have identified many genetic variations associated with type 2 diabetes mellitus (T2DM) in Asians, but understanding the functional genetic variants that influence traits is often a complex process. In this study, fine mapping and other analytical strategies were performed to investigate the effects of G protein signaling modulator 1 (GPSM1) on insulin resistance in skeletal muscle. A total of 128 single-nucleotide polymorphisms (SNPs) within GPSM1 were analysed in 21,897 T2DM cases and 32,710 healthy controls from seven GWASs. The SNP rs28539249 in intron 9 of GPSM1 showed a nominally significant association with T2DM in Asians (OR = 1.07, 95% CI = 1.04–1.10, P < 10−4). The GPSM1 mRNA was increased in skeletal muscle and correlated with T2DM traits across obese mice model. An eQTL for the cis-acting regulation of GPSM1 expression in human skeletal muscle was identified for rs28539249, and the increased GPSM1 expression related with T2DM traits within GEO datasets. Another independent Asian cohort showed that rs28539249 is associated with the skeletal muscle expression of CACFD1, GTF3C5, SARDH, and FAM163B genes, which are functionally enriched for endoplasmic reticulum stress (ERS) and unfolded protein response (UPR) pathways. Moreover, rs28539249 locus was predicted to disrupt regulatory regions in human skeletal muscle with enriched epigenetic marks and binding affinity for CTCF. Supershift EMSA assays followed luciferase assays demonstrated the CTCF specifically binding to rs28539249-C allele leading to decreased transcriptional activity. Thus, the post-GWAS annotation confirmed the Asian-specific association of genetic variant in GPSM1 with T2DM, suggesting a role for the variant in the regulation in skeletal muscle.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Summary statistics of two Japanese GWAS (study 1: 9817 cases, 6763 controls; study 2: 5646 cases, 19,420 controls) for directly genotyped data are available through the NBDC Human Database website (http://humandbs.biosciencedbc.jp/en/).
References
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–46.
Huber CA, Schwenkglenks M, Rapold R, Reich O. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord. 2014;14:44.
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31.
Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44:659–69.
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16.
Hara K, Fujita H, Johnson TA, Yamauchi T, Yasuda K, Horikoshi M, et al. Genome-wide association study identifies three novel loci for type 2 diabetes. Hum Mol Genet. 2014;23:239–46.
Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005.
Huyghe JR, Jackson AU, Fogarty MP, Buchkovich ML, Stancakova A, Stringham HM, et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45:197–201.
Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–15.
Scott LJ, Erdos MR, Huyghe JR, Welch RP, Beck AT, Wolford BN, et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat Commun. 2016;7:11764.
Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73.
The International HapMap Project. Nature. 2003;426:789–96.
Below JE, Parra EJ, Gamazon ER, Torres J, Krithika S, Candille S, et al. Meta-analysis of lipid-traits in Hispanics identifies novel loci, population-specific effects, and tissue-specific enrichment of eQTLs. Sci Rep. 2016;6:19429.
Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5.
Sim X, Ong RT, Suo C, Tay WT, Liu J, Ng DP, et al. Transferability of type 2 diabetes implicated loci in multi-ethnic cohorts from Southeast Asia. PLoS Genet. 2011;7:e1001363.
Shu XO, Long J, Cai Q, Qi L, Xiang YB, Cho YS, et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010;6:e1001127.
Imamura M, Takahashi A, Yamauchi T, Hara K, Yasuda K, Grarup N. et al. Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nature communications. 2016;7:10531.
Al Safar HS, Cordell HJ, Jafer O, Anderson D, Jamieson SE, Fakiola M, et al. A genome-wide search for type 2 diabetes susceptibility genes in an extended Arab family. Ann Hum Genet. 2013;77:488–503.
Alsafar H, Jama-Alol KA, Hassoun AAK, Tay GK. The prevalence of Type 2 diabetes mellitus in the United Arab Emirates: justification for the establishment of the Emirates Family Registry. Int J Diabetes Dev Ctries. 2012;32:25–32.
Tan ALM, Langley SR, Tan CF, Chai JF, Khoo CM, Leow MK, et al. Ethnicity-specific skeletal muscle transcriptional signatures and their relevance to insulin resistance in Singapore. J Clin Endocrinol Metab. 2019;104:465–86.
Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–8.
Handen A, Ganapathiraju MK. LENS: web-based lens for enrichment and network studies of human proteins. BMC Med Genomics. 2015;8 Suppl 4:S2.
Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016;44:W83–9.
Gene Ontology Consortium. going forward. Nucleic Acids Res. 2015;43:D1049–56.
Peng WX, He RZ, Zhang Z, Yang L, Mo YY. LINC00346 promotes pancreatic cancer progression through the CTCF-mediated Myc transcription. Oncogene. 2019;38:6770–80.
Pizzinat N, Takesono A, Lanier SM. Identification of a truncated form of the G-protein regulator AGS3 in heart that lacks the tetratricopeptide repeat domains. J Biol Chem. 2001;276:16601–10.
Sato M, Blumer JB, Simon V, Lanier SM. Accessory proteins for G proteins: partners in signaling. Annu Rev Pharm Toxicol. 2006;46:151–87.
Blumer JB, Cismowski MJ, Sato M, Lanier SM. AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharm Sci. 2005;26:470–6.
Saxena R, Saleheen D, Been LF, Garavito ML, Braun T, Bjonnes A, et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in Sikhs of Punjabi origin from India. Diabetes. 2013;62:1746–55.
Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2011;44:67–72.
Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211.
Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–96.
Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, Attar-Cohen H, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325:1246–50.
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8.
Gao JR, Qin XJ, Jiang H, Wang T, Song JM, Xu SZ. The effects of Qi Teng Xiao Zhuo granules, traditional Chinese medicine, on the expression of genes in chronic glomerulonephritis rats. J Ethnopharmacol. 2016;193:140–49.
Lin J, Hu Y, Nunez S, Foulkes AS, Cieply B, Xue C, et al. Transcriptome-wide analysis reveals modulation of human macrophage inflammatory phenotype through alternative splicing. Arterioscler Thromb Vasc Biol. 2016;36:1434–47.
Hsieh YJ, Wang Z, Kovelman R, Roeder RG. Cloning and characterization of two evolutionarily conserved subunits (TFIIIC102 and TFIIIC63) of human TFIIIC and their involvement in functional interactions with TFIIIB and RNA polymerase III. Mol Cell Biol. 1999;19:4944–52.
Wang C, Ha X, Li W, Xu P, Zhang Z, Wang T, et al. Comparative gene expression profile and DNA methylation status in diabetic patients of Kazak and Han people. Medicine. 2018;97:e11982.
Porter DH, Cook RJ, Wagner C. Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch Biochem Biophys. 1985;243:396–407.
Gong J, Du X, Li Z, Li X, Guo M, Lu J, et al. Differential expression of genes identified by suppression subtractive hybridization in liver and adipose tissue of gerbils with diabetes. PLoS ONE. 2018;13:e0191212.
Ivarsson Y, Arnold R, McLaughlin M, Nim S, Joshi R, Ray D, et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc Natl Acad Sci USA. 2014;111:2542–7.
Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceschini N, Ardissino D, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7.
Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61.
Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425.
Kawamoto E, Koshinaka K, Yoshimura T, Masuda H, Kawanaka K. Immobilization rapidly induces muscle insulin resistance together with the activation of MAPKs (JNK and p38) and impairment of AS160 phosphorylation. Physiol Rep. 2016;4:e12876.
Acknowledgements
We thank all the researchers for their contribution and support for making this study possible. In particular, we would like to thank Anne U. Jackson and Michael Boehnke for Finland GWAS data offered. This work was supported by grants from National Science Foundation of China (No. 181570714 and 8161113013).
Author information
Authors and Affiliations
Contributions
QD: Data extraction, data analysis, and interpretation and paper writing and figure preparation. ALMT: Experiment performance and data analysis, and interpretation and paper writing and figure preparation. EJP, MC, XS, YYT, JL and HA: Data provision and data interpretation. EP and EST: Study design, data interpretation, and paper writing. HC: Study design, data interpretation, manuscript writing, and drawing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ding, Q., Tan, A.L.M., Parra, E.J. et al. Genome-wide meta-analysis associates GPSM1 with type 2 diabetes, a plausible gene involved in skeletal muscle function. J Hum Genet 65, 411–420 (2020). https://doi.org/10.1038/s10038-019-0720-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-019-0720-3