Abstract
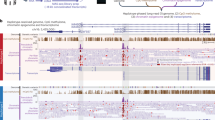
Whole-exome sequencing (WES) can detect not only single-nucleotide variants in causal genes, but also pathogenic copy-number variations using several methods. However, there may be overlooked pathogenic variations in the out of target genome regions of WES analysis (e.g., promoters), leaving many patients undiagnosed. Whole-genome sequencing (WGS) can potentially analyze such regions. We applied long-read nanopore WGS and our recently developed analysis pipeline “dnarrange” to a patient who was undiagnosed by trio-based WES analysis, and identified a heterozygous 97-kb deletion partially involving 5′-untranslated exons of MBD5, which was outside the WES target regions. The phenotype of the patient, a 32-year-old male, was consistent with haploinsufficiency of MBD5. The transcript level of MBD5 in the patient’s lymphoblastoid cells was reduced. We therefore concluded that the partial MBD5 deletion is the culprit for this patient. Furthermore, we found other rare structural variations (SVs) in this patient, i.e., a large inversion and a retrotransposon insertion, which were not seen in 33 controls. Although we considered that they are benign SVs, this finding suggests that our pipeline using long-read WGS is useful for investigating various types of potentially pathogenic SVs. In conclusion, we identified a 97-kb deletion, which causes haploinsufficiency of MBD5 in a patient with neurodevelopmental disorder, demonstrating that long-read WGS is a powerful technique to discover pathogenic SVs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–55.
Tennessen JA, Bigham AW, O’Connor TD, Fu W, Kenny EE, Gravel S, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–9.
Fromer M, Moran JL, Chambert K, Banks E, Bergen SE, Ruderfer DM, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet. 2012;91:597–607.
Miyatake S, Koshimizu E, Fujita A, Fukai R, Imagawa E, Ohba C, et al. Detecting copy-number variations in whole-exome sequencing data using the eXome Hidden Markov Model: an ‘exome-first’ approach. J Hum Genet. 2015;60:175–82.
Nord AS, Lee M, King M-C, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genom. 2011;12:184.
Kosugi S, Momozawa Y, Liu X, Terao C, Kubo M, Kamatani Y. Comprehensive evaluation of structural variation detection algorithms for whole genome sequencing. Genome Biol. 2019;20:117.
Sone J, Mitsuhashi S, Fujita A, Mizuguchi T, Hamanaka K, Mori K, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51:1215–21.
Mizuguchi T, Suzuki T, Abe C, Umemura A, Tokunaga K, Kawai Y, et al. A 12-kb structural variation in progressive myoclonic epilepsy was newly identified by long-read whole-genome sequencing. J Hum Genet. 2019;64:359–68.
Cretu Stancu M, van Roosmalen MJ, Renkens I, Nieboer MM, Middelkamp S, de Ligt J, et al. Mapping and phasing of structural variation in patient genomes using nanopore sequencing. Nat Commun. 2017;8:1326.
Mitsuhashi S, Ohori S, Katoh K, Frith MC, Matsumoto N. A pipeline for complete characterization of complex germline rearrangements from long DNA reads. Genome Med. 2020;12:67.
Lei M, Liang D, Yang Y, Mitsuhashi S, Katoh K, Miyake N, et al. Long-read DNA sequencing fully characterized chromothripsis in a patient with Langer–Giedion syndrome and Cornelia de Lange syndrome-4. J Hum Genet. 2020;65:667–74.
Wagenstaller J, Spranger S, Lorenz-Depiereux B, Kazmierczak B, Nathrath M, Wahl D, et al. Copy-number variations measured by single-nucleotide-polymorphism oligonucleotide arrays in patients with mental retardation. Am J Hum Genet. 2007;81:768–79.
Talkowski ME, Mullegama SV, Rosenfeld JA, van Bon BWM, Shen Y, Repnikova EA, et al. Assessment of 2q23.1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder. Am J Hum Genet. 2011;89:551–63.
Bonnet C, Ali Khan A, Bresso E, Vigouroux C, Béri M, Lejczak S, et al. Extended spectrum of MBD5 mutations in neurodevelopmental disorders. Eur J Hum Genet. 2013;21:1457–61.
Mullegama SV, Rosenfeld JA, Orellana C, van Bon BWM, Halbach S, Repnikova EA, et al. Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder. Eur J Hum Genet. 2014;22:57–63.
Mullegama SV, Elsea SH. Clinical and Molecular Aspects of MBD5-Associated Neurodevelopmental Disorder (MAND). Eur J Hum Genet. 2016;24:1235–43.
Sakamoto M, Kouhei D, Haniffa M, Silva S, Troncoso M, Santander P, et al. A novel ITPA variant causes epileptic encephalopathy with multiple-organ dysfunction. J Hum Genet. 2020;65:751–7.
Tsuchida N, Nakashima M, Kato M, Heyman E, Inui T, Haginoya K, et al. Detection of copy number variations in epilepsy using exome data. Clin Genet. 2018;93:577–87.
MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The database of genomic variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42:D986–92.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Guernsey D, Matsuoka M, Jiang H, Evans S, Macgillivray C, Nightingale M, et al. Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat Genet. 2011;43:360–4.
Mills RE, Bennett EA, Iskow RC, Devine SE. Which transposable elements are active in the human genome? Trends Genet: TIG. 2007;23:183–91.
Kvikstad EM, Piazza P, Taylor JC, Lunter G. A high throughput screen for active human transposable elements. BMC Genom. 2018;19:115.
Stewart C, Kural D, Strömberg MP, Walker JA, Konkel MK, Stütz AM, et al. A comprehensive map of mobile element insertion polymorphisms in humans. PLoS Genet. 2011;7:e1002236.
Karaca E, Harel T, Pehlivan D, Jhangiani SN, Gambin T, Coban Akdemir Z, et al. Genes that affect brain structure and function identified by rare variant analyses of Mendelian neurologic disease. Neuron. 2015;88:499–513.
Guo H, Duyzend MH, Coe BP, Baker C, Hoekzema K, Gerdts J, et al. Genome sequencing identifies multiple deleterious variants in autism patients with more severe phenotypes. Genet Med: Off J Am Coll Med Genet. 2019;21:1611–20.
Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22:245–57.
Mullegama SV, Elsea SH. Intragenic MBD5 familial deletion variant does not negatively impact MBD5 mRNA expression. Mol Cytogenet. 2014;7:80.
Fishilevich S, Nudel R, Rappaport N, Hadar R, Plaschkes I, Iny Stein T, et al. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database: J Biol Databases Curation. 2017;2017.
Aneichyk T, Hendriks WT, Yadav R, Shin D, Gao D, Vaine CA, et al. Dissecting the causal mechanism of X-linked dystonia-parkinsonism by integrating genome and transcriptome assembly. Cell. 2018;172:897–909.e21.
Gonçalves A, Oliveira J, Coelho T, Taipa R, Melo-Pires M, Sousa M, et al. Exonization of an intronic LINE-1 element causing becker muscular dystrophy as a novel mutational mechanism in dystrophin gene. Genes (Basel). 2017;8.
Acknowledgements
We thank all patients and family members for participating in this study. This work was supported by Japan Agency for Medical Research and Development (AMED) under Grant numbers JP20ek0109486, JP20dm0107090, JP20ek0109301, JP20ek0109348, JP20kk0205012 (NM); JSPS KAKENHI under Grant numbers JP17H01539 (NM), JP20K08164 (TM); intramural research grants for Neurological and Psychiatric Disorders of NCNP from the Ministry of Health, Labor and Welfare under Grant numbers 30-6 (NM) and 30-7 (NM), and the Takeda Science Foundation (TM and NM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ohori, S., Tsuburaya, R.S., Kinoshita, M. et al. Long-read whole-genome sequencing identified a partial MBD5 deletion in an exome-negative patient with neurodevelopmental disorder. J Hum Genet 66, 697–705 (2021). https://doi.org/10.1038/s10038-020-00893-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-020-00893-8
This article is cited by
-
Mosaic deletions detected by genome sequencing in two families
Journal of Human Genetics (2025)
-
Combining optical genome mapping and RNA-seq for structural variants detection and interpretation in unsolved neurodevelopmental disorders
Genome Medicine (2024)
-
A novel tandem duplication of PRDM13 in a Chinese family with North Carolina macular dystrophy
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Long-read sequencing reveals the structural complexity of genomic integration of HBV DNA in hepatocellular carcinoma
npj Genomic Medicine (2021)