Abstract
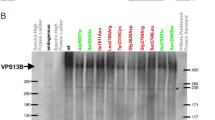
The clinical diagnosis of patients with multisystem involvement including a pronounced neurologic damage is challenging. High-throughput sequencing methods remains crucial to provide an accurate diagnosis. In this study, we reported a Tunisian patient manifesting hypotonia and global developmental delay with visual and skin abnormalities. Exome sequencing was conducted followed by segregation analysis and, subsequently additional investigations. In silico analysis of non-synonymous variants (nsSNPs) described in COG5 in conserved positions was made. Results revealed a homozygous missense variant c.298 C > T (p.Leu100Phe) in the COG5 inherited from both parents. This variant altered both protein solubility and stability, in addition to a putative disruption of the COG5-COG7 interaction. This disruption has been confirmed using patient-derived cells in vitro in a COG5 co-immuno-precipitation, where interaction with binding partner COG7 was abrogated. Hence, we established the COG5-CDG diagnosis. Clinically, the patient shared common features with the already described cases with the report of the ichtyosis as a new manifestation. Conversely, the CADD scoring revealed 19 putatively pathogenic nsSNPs (Minor Allele Frequency MAF < 0.001, CADD > 30), 11 of which had a significant impact on the solubility and/or stability of COG5. These properties seem to be disrupted by six of the seven missense COG5-CDG variants. In conclusion, our study expands the genetic and phenotypic spectrum of COG5-CDG disease and highlight the utility of the next generation sequencing as a powerful tool in accurate diagnosis. Our results shed light on a likely molecular mechanism underlying the pathogenic effect of missense COG5 variants, which is the alteration of COG5 stability and solubility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jaeken J, Matthijs G. Congenital disorders of glycosylation: a rapidly expanding disease family. Annu Rev Genomics Hum Genet. 2007;8:261–78.
Jaeken J, Hennet T, Matthijs G, Freeze HH. CDG nomenclature: time for a change! Biochimica et Biophysica Acta. 2009;1792:825–6.
Péanne R, De Lonlay P, Foulquier F, Kornak U, Lefeber DJ, Morava E, et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur J Med Genet. 2008;61:643–63.
Ondruskova N, Cechova A, Hansikova H, Honzik T, Jaeken J. Congenital disorders of glycosylation: Still “hot” in 2020. Biochimica et Biophysica Acta (BBA)-General Subjects. 2021;1865:129751.
Pokrovskaya ID, Willett R, Smith RD, Morelle W, Kudlyk T, Lupashin VV. Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery. Glycobiology. 2011;21:1554–69.
Rymen D, Keldermans L, Race V, Régal L, Deconinck N, Dionisi-Vici C, et al. COG5-CDG: expanding the clinical spectrum. Orphanet J Rare Dis. 2012;7:1–10.
González-Domínguez CA, Raya-Trigueros A, Manrique-Hernández S, González Jaimes A, Salinas-Marín R, Molina-Garay C, et al. Identification through exome sequencing of the first PMM2-CDG individual of Mexican mestizo origin. Mol Genet Metab Rep. 2020;25:100637.
Matthijs G, Rymen D, Millón MBB, Souche E, Race V. Approaches to homozygosity mapping and exome sequencing for the identification of novel types of CDG. Glycoconj J. 2013;30:67–76.
Zhang Z, Huang TL, Ma J, He WJ, Gu H. Clinical and whole-exome sequencing findings in two siblings from Hani ethnic minority with congenital glycosylation disorders. BMC Med Genet. 2019;20:1–6.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–23.
Oka T, Vasile E, Penman M, Novina CD, Dykxhoorn DM, Ungar D, et al. Genetic analysis of the subunit organization and function of the conserved oligomeric golgi (COG) complex. J Biol Chem 2005;280:32736–45.
Gudmundsson S, Singer‐Berk M, Watts NA, Phu W, Goodrich JK, Solomonson M, et al. Variant interpretation using population databases: Lessons from gnomAD. Hum Mutat 2022;43:1012–30.
Loh E, Hong W. The binary interacting network of the conserved oligomeric Golgi tethering complex. J Biol Chem. 2004;279:24640–8.
Ha JY, Pokrovskaya ID, Climer LK, Shimamura GR, Kudlyk T, Jeffrey PD, et al. Cog5–Cog7 crystal structure reveals interactions essential for the function of a multisubunit tethering complex. Proc Natl Acad Sci 2014;111:15762–7.
Fung CW, Matthijs G, Sturiale L, Garozzo D, Wong KY, et al. COG5-CDG with a mild neurohepatic presentation. JIMD Rep.-Case Res Rep. 2012;2011:67–70.
Paesold-Burda P, Maag C, Troxler H, Foulquier F, Kleinert P, Schnabel S, et al. Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum Mol Genet 2009;18:4350–6.
Chérot E, Keren B, Dubourg C, Carré W, Fradin M, Lavillaureix A, et al. Using medical exome sequencing to identify the causes of neurodevelopmental disorders: experience of 2 clinical unit and 216 patients. Clin Genet. 2018;93:567–76.
Elmas M, Yıldız H, Erdoğan M, Gogus B, Avcı K, Solak M. Comparison of clinical parameters with whole exome sequencing analysis results of autosomal recessive patients; a center experience. Mol Biol Rep. 2019;46:287–99.
Wang X, Han L, Wang XY, Wang JH, Li XM, Jin CH, et al. Identification of two novel mutations in COG5 causing congenital disorder of glycosylation. Front Genet 2020;11:168.
Yin S, Gong L, Qiu H, Zhao Y, Zhang Y, Liu C, et al. Novel compound heterozygous COG5 mutations in a Chinese male patient with severe clinical symptoms and type IIi congenital disorder of glycosylation: a case report. Experimental and Therapeutic. Medicine. 2019;18:2695–2700.
Ferrer A, Starosta RT, Ranatunga W, Ungar D, Kozicz T, Klee E, Rust LM, et al. Fetal glycosylation defect due to ALG3 and COG5 variants detected via amniocentesis: Complex glycosylation defect with embryonic lethal phenotype. Mol Genet Metab. 2020;100:424–9.
Stavropoulos DJ, Merico D, Jobling R, Bowdin S, Monfared N, Thiruvahindrapuram B, et al. Whole-genome sequencing expands diagnostic utility and improves clinical management in paediatric medicine. NPJ Genom Med. 2016;1:1–9.
Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2010;291:2370–6.
Redler RL, Das J, Diaz JR, Dokholyan NV. Protein destabilization as a common factor in diverse inherited disorders. J Mol Evolut. 2016;82:11–16.
Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, Vendruscolo M. Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 2013;5:781–90.
Acknowledgements
We would like to thank the studied family for the cooperation to perform the present study. We are also grateful to the entire team of Child neurology departement in the CHU Hedi Chaker of Sfax for their help and availability.
Author information
Authors and Affiliations
Contributions
BK: conceptualization, methodology, formal analysis, interpretation of data, writing original draft, review & editing; US: elaborating the functional analysis, review& editing; ABI, WB : Contributed to the collection and the analysis of clinical data, ES-R, AYJN, CB : contributed with reagents/materials necessary for the functional analysis, reviewed the final draft of the manuscript, HM : design of work, interpretation of data, reviewed the final draft of the manuscript, IZ : Performed and analyzed the Modeling study, FF: contributed with reagents/materials necessary for the segregation analysis, reviewed the final draft of the manuscript, FK: contributed with the analysis of the clinical data, BR : performing and interpretation of the WES data, supervising the functional study, reviewed the final draft of the manuscript, CCT : conceived and designed the study, supervise data analysis and reviewed drafts of the paper. All authors have approved the manuscript and agree with the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The study was conducted according to the guidelines ofthe Declaration of Helsink and approved by the Regional ethics Committee of the Protection of Persons, Sfax, Tunisia (CPP SUD) (number 0193/2019).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khabou, B., Sahari, U.B.M., ben Issa, A. et al. Characterization of a missense variant in COG5 in a Tunisian patient with COG5-CDG syndrome and insights into the effect of non-synonymous variants on COG5 protein. J Hum Genet 69, 591–597 (2024). https://doi.org/10.1038/s10038-024-01273-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-024-01273-2