Abstract
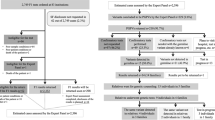
We surveyed the status of the secondary finding (SF) disclosure in comprehensive genome profiling (CGP) in 2020. The situation has changed: increase in the number of hospitals that provide CGP, an update to the Comprehensive Tumor Genomic Profiling: Materials for Review of Secondary Findings (CTGPMRSF), and the addition of a liquid biopsy test, FoundationOne® Liquid CDx (F1L). Moreover, the actual situation was unclear because the 2020 survey did not include all designated and cooperative hospitals. Herein, we conducted a questionnaire survey of all designated-core, designated, and cooperative hospitals to identify the current status and challenges concerning SF in the CGP in 2022. A total of 82.1% of the hospitals responded and 77.7% of the response was from cooperative hospitals. Approximately 80% of the hospitals used CTGPMRSF. SF disclosure, confirmatory test implementation, and SF confirmation rates were 12.4%, 31.6%, and 46.6% for FoundationOne® CDx (F1CDx), respectively, and 6.8%, 31.8%, and 70.7% for F1L, respectively. The implementation rate of the confirmatory test was substantially higher in hospitals with genetic experts and in hospitals that could conduct confirmatory tests on the same day. Our survey provides insight into how SF is handled in Japan. The percentage of cases leading to confirmatory tests has gradually increased, although challenges such as insurance coverage limitations and varied understanding of SF among patients and healthcare providers persist. With the increasing use of whole-genome analysis, our findings will provide valuable insights into establishing an effective SF disclosure system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Minamoto A, Yamada T, Shimada S, Kinoshita I, Aoki Y, Oda K, et al. Current status and issues related to secondary findings in the first public insurance covered tumor genomic profiling in Japan: multi-site questionnaire survey. J Hum Genet. 2022;67:557–63. https://doi.org/10.1038/s10038-022-01028-x
Shimada S, Yamada T, Iwakuma M, Kosugi S. Physicians’ perceptions of the factors influencing disclosure of secondary findings in tumour genomic profiling in Japan: a qualitative study. Eur J Hum Genet. 2022;30:88–94. https://doi.org/10.1038/s41431-021-00944-4
Kawamura M, Shirota H, Niihori T, Komine K, Takahashi M, Takahashi S, et al. Management of patients with presumed germline pathogenic variant from tumor-only genomic sequencing: a retrospective analysis at a single facility. J Hum Genet. 2023;68:399–408. https://doi.org/10.1038/s10038-023-01133-5
Kage H, Oda K, Muto M, Tsuchihara K, Okita N, Okuma Y, et al. Human resources for administrative work to carry out a comprehensive genomic profiling test in Japan. Cancer Sci. 2023;114:3041–9. https://doi.org/10.1111/cas.15833
Kikuchi J, Ohhara Y, Takada K, Tanabe H, Hatanaka K, Amano T, et al. Clinical significance of comprehensive genomic profiling tests covered by public insurance in patients with advanced solid cancers in Hokkaido, Japan. Jpn J Clin Oncol. 2021;51:753–61. https://doi.org/10.1093/jjco/hyaa277. Erratum in: Jpn J Clin Oncol. 2022;52:1455
Sunami K, Naito Y, Aimono E, Amano T, Ennishi D, Kage H, et al. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int J Clin Oncol. 2021;26:443–9. https://doi.org/10.1007/s10147-020-01844-1. Epub 2021 Jan 1. Erratum in: Int J Clin Oncol. 2021;26:1007
Sunami K, Naito Y, Komine K, Amano T, Ennishi D, Imai M, et al. Chronological improvement in precision oncology implementation in Japan. Cancer Sci. 2022;113:3995–4000. https://doi.org/10.1111/cas.15517
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. https://doi.org/10.1136/bmj.39335.541782.AD
O’Cathain A, Thomas KJ. “Any other comments?” Open questions on questionnaires—a bane or a bonus to research? BMC Med Res Methodol. 2004;4:25 https://doi.org/10.1186/1471-2288-4-25
Braun V, Clarke V. Successful qualitative research: a practical guide for beginners. London: SAGE Publications; 2013.
Tung N, Dougherty KC, Gatof ES, DeLeonardis K, Hogan L, Tukachinsky H, et al. Potential pathogenic germline variant reporting from tumor comprehensive genomic profiling complements classic approaches to germline testing. NPJ Precis Oncol. 2023;7:76 https://doi.org/10.1038/s41698-023-00429-1
Schrader KA, Cheng DT, Joseph V, Prasad M, Walsh M, Zehir A, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–11. https://doi.org/10.1001/jamaoncol.2015.5208. Erratum in: JAMA Oncol. 2016;2(2):279. Doi: 10.1001/jamaoncol.2015.6541. Hyman, David [corrected to Hyman, David M]
Cobain EF, Wu YM, Vats P, Chugh R, Worden F, Smith DC. et al.Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors.JAMA Oncol.2021;7:525–33. https://doi.org/10.1001/jamaoncol.2020.7987
Stadler ZK, Maio A, Chakravarty D, Kemel Y, Sheehan M, Salo-Mullen E, et al. Therapeutic implications of germline testing in patients with advanced cancers. J Clin Oncol. 2021;39:2698–709. https://doi.org/10.1200/JCO.20.03661
Kuzbari Z, Bandlamudi C, Loveday C, Garrett A, Mehine M, George A, et al. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol. 2023;34:215–27. https://doi.org/10.1016/j.annonc.2022.12.003
Nowlen CJ, Daniels M, Uzunparmak B, Ileana Dumbrava EE, Yuan Y, Patel KP, et al. Limited independent follow-up with germline testing of variants detected in BRCA1 and BRCA2 by tumor-only sequencing. J Immunother Precis Oncol. 2024;7:7–17. https://doi.org/10.36401/JIPO-23-2
Catapano F, El Hachmi M, Ketterer-Heng N, Renieri A, Mari F, Morris M, et al. The role of the Genetic Counsellor in the multidisciplinary team: the perception of geneticists in Europe. Eur J Hum Genet. 2022;30:1432–8. https://doi.org/10.1038/s41431-022-01189-5. Epub 2022 Oct 5. Erratum in: Eur J Hum Genet. 2024;32:133
Aizawa Y, Watanabe A, Kato K. Institutional and social issues surrounding genetic counselors in Japan: current challenges and implications for the global community. Front Genet. 2021;12:646177 https://doi.org/10.3389/fgene.2021.646177
Japanese Board of Medical Genetics and Genomics, Clinical Genetics. List of clinical geneticists [Internet]. Japan; 2024 February 28 [cited 28 Feb 2024]. Available from: https://www.jbmg.jp/senmon/
Japanese Board of Certified Genetic Counselors. List of certified genetic counselors [Internet]. Japan: December 2023 [cited 28 Feb 2024]. Available from: https://plaza.umin.ac.jp/~GC/About.html
Muto K, Nagai A, Ri I, Takashima K, Yoshida S. Is legislation to prevent genetic discrimination necessary in Japan? An overview of the current policies and public attitudes. J Hum Genet. 2023;68:579–85. https://doi.org/10.1038/s10038-023-01163-z. Epub 2023 Jun 8. Erratum in: J Hum Genet. 2023
Ryoshitu katu tekisetu na genomu iryou wo kokumin ga annshinnshite ukerareruyou ni surutame no shisaku no sougouteki katu keikakuteki na suishin ni kannsuru hourituann (Bill for an Act on Comprehensive and Systematic Promotion of Measures to Guarantee the Public’s Reliable Access to High-Quality Appropriate Genomic Medicine with Comfort) Shuho (Bills submitted by Members of House of Representatives) The 211th ordinary session of the Diet §18 (2023). https://www.shugiin.go.jp/internet/itdb_gian.nsf/html/gian/honbun/youkou/g21105018.htm
Ri I, Kawata J, Nagai A, Muto K. Expectations, concerns, and attitudes regarding whole-genome sequencing studies: a survey of cancer patients, families, and the public in Japan. J Hum Genet. 2023;68:281–5. https://doi.org/10.1038/s10038-022-01100-6. Epub 2022 Dec 12. Erratum in: J Hum Genet. 2022
Ministry of Health, Labour and Welfare. Action Plan for Whole Genome Analysis 2022 [document on internet]. Japan; 2022 September 30 [cited 28 Feb 2024]. Available from: https://www.mhlw.go.jp/content/10901000/001073173.pdf
Acknowledgements
We would like to thank all the hospital staff members who took the time to respond to our survey. This study was supported by the Japan Ministry of Health, Labour and Welfare (grant numbers: 20AD1001), the Japan Ministry of Education, Culture, Sports, Science and Technology (22K07469), the Japan Agency for Medical Research and Development (AMED) (17kk0305006h0001), and the National Cancer Center Research and Development Fund (31-A-2, 2022-A-07, 2023-A-18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimada, S., Yamada, T., Minamoto, A. et al. Nationwide survey of the secondary findings in cancer genomic profiling: survey including liquid biopsy. J Hum Genet 70, 33–40 (2025). https://doi.org/10.1038/s10038-024-01294-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-024-01294-x