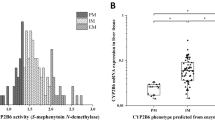
Abstract
Recent studies of animal models reported Nicotinamide N-methyltransferase (NNMT) as a potential therapeutic target for preventing alcohol-associated fatty liver (AFL), yet its efficacy and safety in humans remain unknown. We aim to estimate the effectiveness and safety of inhibiting NNMT in humans. We leveraged Electronic Medical Records (EMRs) data coupled with genetic information to perform a retrospective drug target validation study. We examined longitudinal clinical data from 612 individuals with excessive alcohol consumption. Two variants lowering NNMT protein levels were combined to calculate a weighted NNMT genetic score that could mimic mild inhibition of NNMT. Participants with an NNMT score above the median were classified as genetically inhibited, while others were considered non-inhibited. We then evaluated whether genetic inhibition of NNMT would affect the incidence of AFL or the risk of liver injury, to illuminate the effectiveness and safety of genetic inhibition of NNMT respectively. NNMT genetic inhibition correlated with a reduced AFL risk (hazard ratio [HR] 0.67, 95% confidence interval [CI] 0.49–0.90, P = 0.009) without a significant increase in serum aminotransferase levels (P > 0.10). Notably, elevated ALT and AST levels were observed (P < 0.05) in the genetically inhibited group prior to alcohol exposure. These findings suggest NNMT inhibition is a promising avenue for AFL prevention among individuals with excessive alcohol intake. They also underscore the need for precise target population identification to mitigate potential adverse effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–8.
Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Prim. 2018;4:16.
Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Invest [Internet]. 2024 Feb 1 [cited 2024 Nov 5];134. Available from: https://www.jci.org/articles/view/176345.
Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85.
Pissios P. Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme. Trends Endocrinol Metab. 2017;28:340–53.
Roberti A, Fernández AF, Fraga MF. Nicotinamide N-methyltransferase: at the crossroads between cellular metabolism and epigenetic regulation. Mol Metab. 2021;45:101165.
Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, et al. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:e479348.
Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–77.
Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99.
Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72.
Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–62.
Komatsu M, Kanda T, Urai H, Kurokochi A, Kitahama R, Shigaki S, et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD + metabolism. Sci Rep. 2018;8:8637.
Song Q, Chen Y, Wang J, Hao L, Huang C, Griffiths A, et al. ER stress-induced upregulation of NNMT contributes to alcohol-related fatty liver development. J Hepatol. 2020;73:783–93.
Ding Q, Ma Y, Lai S, Dou X, Li S. NNMT aggravates hepatic steatosis, but alleviates liver injury in alcoholic liver disease. J Hepatol. 2021;74:1248–50.
Sidor K, Jeznach A, Hoser G, Skirecki T. 1-Methylnicotinamide (1-MNA) inhibits the activation of the NLRP3 inflammasome in human macrophages. Int Immunopharmacol. 2023;121:110445.
Biedroń R, Ciszek M, Tokarczyk M, Bobek M, Kurnyta M, Słominska EM, et al. 1-Methylnicotinamide and nicotinamide: two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch Immunol Ther Exp. 2008;56:127–34.
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14.
McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87:162–71.
Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–94.
Koscielny G, An P, Carvalho-Silva D, Cham JA, Fumis L, Gasparyan R, et al. Open Targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 2017;45:D985–94.
Tian Z, Chen F, Wang J, Wu B, Shao J, Liu Z, et al. CAS Array: design and assessment of a genotyping array for Chinese biobanking. Precis Clin Med. 2023;6:pbad002.
Ferkingstad E, Sulem P, Atlason BA, Sveinbjornsson G, Magnusson MI, Styrmisdottir EL, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet. 2021;53:1712–21.
Zhang J, Dutta D, Köttgen A, Tin A, Schlosser P, Grams ME, et al. Plasma proteome analyses in individuals of European and African ancestry identify cis-pQTLs and models for proteome-wide association studies. Nat Genet. 2022;54:593–602.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–45.
Sherman KE. Alanine aminotransferase in clinical practice: a review. Arch Intern Med. 1991;151:260–5.
McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016;15:817–28.
Holmes RS, Duley JA, Algar EM, Mather PB, Rout UK. Biochemical and genetic studies on enzymes of alcohol metabolism: the mouse as a model organism for human studies. Alcohol Alcohol. 1986;21:41–56.
Liu SX, Du YC, Zeng T. A mini-review of the rodent models for alcoholic liver disease: shortcomings, application, and future prospects. Toxicol Res. 2021;10:523–30.
Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8.
Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol-Gastrointest Liver Physiol. 2004;287:G310–4.
Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501.
Monticello TM, Jones TW, Dambach DM, Potter DM, Bolt MW, Liu M, et al. Current nonclinical testing paradigm enables safe entry to First-In-Human clinical trials: The IQ consortium nonclinical to clinical translational database. Toxicol Appl Pharmacol. 2017;334:100–9.
Jakubowski A, Sternak M, Jablonski K, Ciszek-Lenda M, Marcinkiewicz J, Chlopicki S. 1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α. Int Immunopharmacol. 2016;31:98–104.
Taniki N, Nakamoto N, Chu PS, Mikami Y, Amiya T, Teratani T, et al. Intestinal barrier regulates immune responses in the liver via IL-10–producing macrophages. JCI Insight [Internet]. 2018 Jun 21 [cited 2024 Jan 22];3. Available from: https://insight.jci.org/articles/view/91980.
Sarich P, Canfell K, Banks E, Paige E, Egger S, Joshy G, et al. A prospective study of health conditions related to alcohol consumption cessation among 97,852 drinkers aged 45 and over in Australia. Alcohol: Clin Exp Res. 2019;43:710–21.
Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med. 2003;168:1308–11.
Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57:226–30.
Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025.
Palmentieri B, Desio I, Lamura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Digestive Liver Dis. 2006;38:485–9.
Wang J, Lin X, Bloomgarden ZT, Ning G. The Jiangnan diet, a healthy diet pattern for Chinese. [cited 2024 Nov 7]; Available from: https://onlinelibrary.wiley.com/doi/10.1111/1753-0407.13015.
Acknowledgements
This work was funded by the National High Level Hospital Clinical Research Funding (BJ-2024-144) and Kunshan Key Research and Development Project (KS2214). The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
KZ and TX were responsible for study conception and design; BRW contributed to the researched data, discussion, and data interpretation and wrote the manuscript. XW contributed to the discussion, data interpretation, review and editing the manuscript; YP, ZT, PW, JS, YL and RH contributed to the genetic analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First People’s Hospital of Kunshan, Approval No. 2019-03-001-K01. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, B., Weng, X., Pan, Y. et al. Genetic inhibition of nicotinamide N-methyltransferase and prevention of alcohol-associated fatty liver in humans. J Hum Genet 70, 141–146 (2025). https://doi.org/10.1038/s10038-024-01313-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-024-01313-x