Abstract
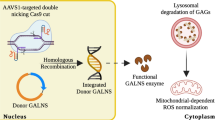
Mucopolysaccharidosis IVA (MPS IVA) is caused by pathogenic variants in the GALNS gene encoding N-acetylgalactosamine-6-sulfate sulfatase (GALNS) enzyme, leading to glycosaminoglycan (GAG) accumulation in multiple tissues, resulting in progressive skeletal dysplasia and poor quality of life. There is currently no effective treatment for this skeletal disease. This study proposes a novel lentiviral vector (LV)-based gene therapy that produces and secretes the active GALNS enzyme at supraphysiologic levels within the cells. LVs carrying the native GALNS encoding sequence (cDNA) were made under three different promoters: CBh, COL2A1, and CD11b. Moreover, we designed LVs carrying the native GALNS cDNA tagged with D8 octapeptide under the CD11b promoter and a human codon-optimized GALNS cDNA under the CBh promoter, respectively. Transduced HEK293 cells, HepG2 cells, and MPS IVA fibroblasts and chondrocytes were cultured for 8 and 30 days, and the media were collected every three days. The enzyme activity, GAG levels, and vector copy numbers (VCNs) in these cells and media were analyzed. LV with the COL2A1 promoter produced the highest enzyme activity in HEK293, HepG2, MPS IVA fibroblasts, and chondrocytes, followed by LV with the CBh promoter. VCNs were higher in MPS IVA fibroblasts treated with LV-CBh-hGALNS and in HepG2 cells treated with LV-CD11b-hGALNS than in HEK293 cells. Accumulated GAGs were normalized to wild-type levels by the LV gene therapy, especially with CBh and COL2A1 promoters. These findings, if further validated, could significantly impact the treatment of MPS IVA, offering a more effective and feasible treatment option.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Celik B, Tomatsu SC, Tomatsu S, Khan SA. Epidemiology of mucopolysaccharidoses update. Diagnostics. 2021;11:273.
HGMD® gene result. GALNS. 2023. Available from: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GALNS.
Peracha H, Sawamoto K, Averill L, Kecskemethy H, Theroux M, Thacker M, et al. Molecular genetics and metabolism, special edition: diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA. Mol Genet Metab. 2018;125:18–37.
Sawamoto K, González JVÁ, Piechnik M, Otero FJ, Couce ML, Suzuki Y, et al. Mucopolysaccharidosis IVA: diagnosis, treatment, and management. Int J Mol Sci. 2020;21. Available from: https://pubmed.ncbi.nlm.nih.gov/32102177/.
Yasuda E, Suzuki Y, Shimada T, Sawamoto K, Mackenzie WG, Theroux MC, et al. Activity of daily living for Morquio A syndrome. Mol Genet Metab. 2016;118:111–22.
Nakamura-Utsunomiya A, Nakamae T, Kagawa R, Karakawa S, Sakata S, Sakura F, et al. A case report of a Japanese boy with Morquio A syndrome: effects of enzyme replacement therapy initiated at the age of 24 months. Int J Mol Sci. 2020;21:989.
Doherty C, Stapleton M, Piechnik M, Mason RW, Mackenzie WG, Yamaguchi S, et al. Effect of enzyme replacement therapy on the growth of patients with Morquio A. J Hum Genet. 2019;64:625–35.
Do Cao J, Wiedemann A, Quinaux T, Battaglia-Hsu SF, Mainard L, Froissart R, et al. 30 months follow-up of an early enzyme replacement therapy in a severe Morquio A patient: about one case. Mol Genet Metab Rep. 2016;9:42–5.
Frigeni M, Rodriguez-Buritica DF, Saavedra H, Gunther KA, Hillman PR, Balaguru D, et al. The youngest pair of siblings with mucopolysaccharidosis type IVA to receive enzyme replacement therapy to date: a case report. Am J Med Genet A. 2021;185:3510–6.
Barak S, Anikster Y, Sarouk I, Stern E, Eisenstein E, Yissar T, et al. Long-term outcomes of early enzyme replacement therapy for mucopolysaccharidosis IV: clinical case studies of two siblings. Diagnostics. 2020;10:108.
Qi Y, Musson DG, Schweighardt B, Tompkins T, Jesaitis L, Shaywitz AJ, et al. Pharmacokinetic and pharmacodynamic evaluation of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome. Clin Pharmacokinet. 2014;53:1137–47.
Tomatsu S, Sawamoto K, Shimada T, Bober MB, Kubaski F, Yasuda E, et al. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin Orphan Drugs. 2015;3:1279–90.
Ponder KP, Immune response hinders therapy for lysosomal storage diseases. J Clin Invest. 2008;118:2686–9.
Long B, Tompkins T, Decker C, Jesaitis L, Khan S, Slasor P, et al. Long-term immunogenicity of elosulfase alfa in the treatment of morquio a syndrome: results from MOR-005, a phase III extension study. Clin Ther. 2017;39:118–129.e3.
Schweighardt B, Tompkins T, Lau K, Jesaitis L, Qi Y, Musson DG, et al. Immunogenicity of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome: results from MOR-004, a phase III trial. Clin Ther. 2015;37:1012–1021.e6.
Melton AC, Soon RK, Tompkins T, Long B, Schweighardt B, Qi Y, et al. Antibodies that neutralize cellular uptake of elosulfase alfa are not associated with reduced efficacy or pharmacodynamic effect in individuals with Morquio A syndrome. J Immunol Methods. 2017;440:41–51.
Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–24.
Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41.
Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, et al. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol Genet Metab. 2016;117:84–94.
Piechnik M, Amendum PC, Sawamoto K, Stapleton M, Khan S, Fnu N, et al. Sex difference leads to differential gene expression patterns and therapeutic efficacy in mucopolysaccharidosis IVA murine model receiving AAV8 gene therapy. Int J Mol Sci. 2022;23:12693.
Wang J, Luan Z, Jiang H, Fang J, Qin M, Lee V, et al. Allogeneic hematopoietic stem cell transplantation in thirty-four pediatric cases of mucopolysaccharidosis-a ten-year report from the China children transplant group. Biol Blood Marrow Transpl. 2016;22:2104–8.
Akyol MU, Alden TD, Amartino H, Ashworth J, Belani K, Berger KI, et al. Recommendations for the management of MPS IVA: systematic evidence- and consensus-based guidance. Orphanet J Rare Dis. 2019;14:137.
Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, et al. Hematopoietic stem cell transplantation for mucopolysaccharidoses: past, present, and future. Biol Blood Marrow Transplant. 2019;25:e226–46.
Sawamoto K, Chen HH, Alméciga-Díaz CJ, Mason RW, Tomatsu S. Gene therapy for Mucopolysaccharidoses. Mol Genet Metab. 2018;123:59.
Sawamoto K, Tomatsu S. Development of substrate degradation enzyme therapy for mucopolysaccharidosis IVA murine model. Int J Mol Sci. 2019;20:4139.
Celik B, Rintz E, Sansanwal N, Khan S, Bigger B, Tomatsu S. Lentiviral vector-mediated ex vivo hematopoietic stem cell gene therapy for mucopolysaccharidosis IVA murine model. Hum Gene Ther. 2024;35:917–37.
Hintze JP, Tomatsu S, Fujii T, Montaño AM, Yamaguchi S, Suzuki Y, et al. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights. 2011;6:69–78.
Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, De Carvalho TG, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010;33:S35–42.
Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, De Carvalho TG, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010 [cited 2023 Feb 1];33SUPPL. 3). Available from: https://pubmed.ncbi.nlm.nih.gov/20107903/.
Khan SA, Mason RW, Giugliani R, Orii K, Fukao T, Suzuki Y, et al. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol Genet Metab. 2018;125:44–52.
Khan SA, Nidhi FNU, Amendum PC, Tomatsu S. Detection of Glycosaminoglycans in Biological Specimens. Methods Mol Biol. 2023;2619:3–24.
Leal AF, Alméciga-Díaz CJ. Efficient CRISPR/Cas9 nickase-mediated genome editing in an in vitro model of mucopolysaccharidosis IVA. Gene Ther. 2023;30:107–114.
Tomatsu S, Montaño AM, Gutierrez M, Grubb JH, Oikawa H, Dung VC, et al. Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol Genet Metab. 2007;91:69–78.
Sawamoto K, Karumuthil-Melethil S, Khan S, Stapleton M, Bruder JT, Danos O, et al. Liver-targeted AAV8 gene therapy ameliorates skeletal and cardiovascular pathology in a mucopolysaccharidosis IVA murine model. Mol Ther Methods Clin Dev [Internet]. 2020;18:50–61.
Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO, et al. Risks associated with lentiviral vector exposures and prevention strategies. J Occup Environ Med. 2016;58:1159–66.
Boulad F, Maggio A, Wang X, Moi P, Acuto S, Kogel F, et al. Lentiviral globin gene therapy with reduced-intensity conditioning in adults with β-thalassemia: a phase 1 trial. Nat Med. 2022;28:63–70.
Alméciga-Díaz CJ, Rueda-Paramo MA, Espejo AJ, Echeverri OY, Montaño A, Tomatsu S, et al. Effect of elongation factor 1α promoter and SUMF1 over in vitro expression of N-acetylgalactosamine-6-sulfate sulfatase. Mol Biol Rep. 2009;36:1863–70.
Zielske SP, Lingas KT, Li Y, Gerson SL. Limited lentiviral transgene expression with increasing copy number in an MGMT selection model: lack of copy number selection by drug treatment. Molecular Ther. 2004;9:923–31.
Corre G, Seye A, Frin S, Ferrand M, Winkler K, Luc C, et al. Lentiviral standards to determine the sensitivity of assays that quantify lentiviral vector copy numbers and genomic insertion sites in cells. Gene Ther. 2022;29:536–43.
Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, et al. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–57.
Puentes-Tellez MA, Sánchez OF, Rojas-Rodriguez F, Benincore-Flóre E, Barbosa H, Alméciga Díaz CJ. Evaluation of HIV–1 derived lentiviral vectors as transductors of Mucopolysaccharidosis type IV a fibroblasts. Gene. 2021;780:145527.
Kos S, Tesic N, Kamensek U, Blagus T, Cemazar M, Kranjc S, et al. Improved specificity of gene electrotransfer to skin using pDNA under the control of collagen tissue-specific promoter. J Membr Biol. 2015;248:919–28.
Karaosmanoğlu O, Banerjee S, Sivas H. Identification of biomarkers associated with partial epithelial to mesenchymal transition in the secretome of slug over-expressing hepatocellular carcinoma cells. Cellular Oncol. 2018;41:439–53.
Bougioukli S, Sugiyama O, Pannell W, Ortega B, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of Bone Morphogenetic Protein 2 –transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29:507–19.
Wright JF. Quantification of CpG motifs in rAAV genomes: avoiding the toll. Molecular Ther. 2020;28:1756–8.
Tomatsu S. Mucopolysaccharidosis IVA: identification of mutations and methylation study in GALNS gene. J Med Genet. 2004;41:e98–e98.
Acknowledgements
BC was supported by the National MPS Society (ID 4892) and the Turkish Ministry of Education (YLSY2017-Doctoral Scholarship). This work was also supported by grants from the Austrian MPS society, A Cure for Robert, Inc., The Carol Ann Foundation, Angelo R. Cali & Mary V. Cali Family Foundation, Inc., The Vain and Harry Fish Foundation, Inc., The Bennett Foundation, Jacob Randall Foundation, and Nemours Funds. ST was supported by an Institutional Development Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NICHD) (1R01HD102545-01A1).
Author information
Authors and Affiliations
Contributions
Conceptualization, BC; methodology, BC; software, BC; validation, BC, AFL, SK, and ST; formal analysis, BC; investigation, BC, AFL, SK; resources, ST; data curation, BC, and ST; writing—original draft preparation, BC; writing—review and editing, BC; visualization, BC; supervision, ST; project administration, BC, and ST; funding acquisition, ST. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Celik, B., Leal, A.F., Khan, S. et al. Assessment of different promoters in lentiviral vectors for expression of the N-acetyl-galactosamine-6-sulfate sulfatase gene. J Hum Genet 70, 463–473 (2025). https://doi.org/10.1038/s10038-025-01353-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-025-01353-x