Abstract
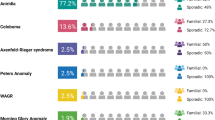
Anophthalmia/microphthalmia (A/M) are rare congenital ocular malformations involving the absence or underdevelopment of the eyes, and they display considerable clinical and genetic heterogeneity. Establishing a genetic diagnosis for A/M is critical because it facilitates early intervention, informed genetic counseling, and the prevention of disease transmission in high-risk families. This study explored the genotypic and phenotypic landscape of A/M in 10 Pakistani families meeting specific criteria: confirmed A/M phenotype, residence in Khyber Pakhtunkhwa, no prior genetic testing, and informed consent. Whole-exome sequencing (WES) and segregation analysis in families identified a novel missense variant in SMOC1 (c.406T>G, p.Cys136Gly) in a family with Waardenburg anophthalmia syndrome (WAS). Additionally, causative variants in VSX2 (c.598C>T, p.Arg200Ter) and ALDH1A3 (c.172dup, p.Glu58GlyfsTer5) were detected, potentially representing founder variants in the Pashtun ethnic group. Moreover, a likely pathogenic variant in FOXE3 (c.145G>T, p.Gly49Ter) and a variant of uncertain significance in STRA6 (c.1399C>T, p.Arg467Cys), which exhibited incomplete penetrance, were also identified. In addition, segregation analysis of the causal genetic variants in the 5 families revealed a carrier frequency of 60.86% among the phenotypically unaffected family members. Notably, the average size of autozygous regions among probands was substantial (282.62 Mb), indicating a high degree of consanguinity and familial relatedness due to endogamous practices. However, no causative variants were identified in five families, each with a single affected member, with unilateral A/M in the majority of cases. These findings support the value of genetic diagnostics in reproductive counseling and highlight the utility of broader genomic approaches to improve diagnostic outcomes in unresolved cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available in the ClinVar repository, with the accession numbers i.e. SCV005901551, SCV005901552, SCV005901553, SCV005901554 and SCV005901555.
References
Basharat R, Rodenburg K, Rodríguez-Hidalgo M, Jarral A, Ullah E, Corominas J, et al. Combined single gene testing and genome sequencing as an effective diagnostic approach for anophthalmia and microphthalmia patients. Genes. 2023;14:1573.
Kuang L, Zhang M, Wang T, Huang T, Li J, Gan R, et al. The molecular genetics of anterior segment dysgenesis. Exp Eye Res. 2023;234:109603.
Slavotinek A. Genetics of anophthalmia and microphthalmia. Part 2: syndromes associated with anophthalmia-microphthalmia. Hum Genet. 2019;138:831–46.
Williamson KA, FitzPatrick DR. The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur J Med Genet. 2014;57:369–80.
Riaz M, Tiller J, Ajmal M, Azam M, Qamar R, Lacaze P. Implementation of public health genomics in Pakistan. Eur J Hum Genet. 2019;27:1485.
Harding P, Moosajee M. The molecular basis of human anophthalmia and microphthalmia. J Dev Biol. 2019;7:16.
Amlie-Wolf L, Bardakjian T, Kopinsky SM, Reis LM, Semina EV, Schneider A. Review of 37 patients with SOX2 pathogenic variants collected by the anophthalmia/microphthalmia clinical registry and DNA research study. Am J Med Genet A. 2022;188:187–98.
Li J, Yang W, Wang YJ, Ma C, Curry CJ, McGoldrick D, et al. Exome sequencing identifies genetic variants in anophthalmia and microphthalmia. Am J Med Genet A. 2022;188:2376.
Lin S, Harlalka GV, Hameed A, Reham HM, Yasin M, Muhammad N, et al. Novel mutations in ALDH1A3 associated with autosomal recessive anophthalmia/microphthalmia, and review of the literature. BMC Med Genet. 2018;19:160.
Tüzmen Ş, Baskın Y, Nursal AF, Eraslan S, Esemen Y, Çalıbaşı G, et al. Techniques for nucleic acid engineering: The foundation of gene manipulation. In: Omics technologies and bio-engineering. Elsevier; 2018. p. 247–315.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164–e164.
Boeva V, Popova T, Bleakley K, Chiche P, Cappo J, Schleiermacher G, et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinform. 2012;28:423–5.
Quinodoz M, Peter VG, Bedoni N, Royer Bertrand B, Cisarova K, Salmaninejad A, et al. AutoMap is a high performance homozygosity mapping tool using next-generation sequencing data. Nat Commun. 2021;12:518.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics Med. 2015;17:405–23.
Slavotinek AM, Garcia ST, Chandratillake G, Bardakjian T, Ullah E, Wu D, et al. Exome sequencing in 32 patients with anophthalmia/microphthalmia and developmental eye defects. Clin Genet. 2015;88:468–73.
Deml B, Reis LM, Lemyre E, Clark RD, Kariminejad A, Semina EV. Novel mutations in PAX6, OTX2 and NDP in anophthalmia, microphthalmia and coloboma. Eur J Hum Genet. 2016;24:535–41.
Haug P, Koller S, Maggi J, Lang E, Feil S, Wlodarczyk A, et al. Whole exome sequencing in coloboma/microphthalmia: identification of novel and recurrent variants in seven genes. Genes. 2021;12:65.
Matías-Pérez D, García-Montaño LA, Cruz-Aguilar M, García-Montalvo IA, Nava-Valdéz J, Barragán-Arevalo T, et al. Identification of novel pathogenic variants and novel gene-phenotype correlations in Mexican subjects with microphthalmia and/or anophthalmia by next-generation sequencing. J Hum Genet. 2018;63:1169–80.
Iseri SU, Wyatt AW, Nürnberg G, Kluck C, Nürnberg P, Holder GE, et al. Use of genome-wide SNP homozygosity mapping in small pedigrees to identify new mutations in VSX2 causing recessive microphthalmia and a semidominant inner retinal dystrophy. Hum Genet. 2010;128:51–60.
Ullah E, Saqib MAN, Sajid S, Shah N, Zubair M, Khan MA, et al. Genetic analysis of consanguineous families presenting with congenital ocular defects. Exp Eye Res. 2016;146:163–71.
Percin EF, Ploder L, Yu J, Arici K, Horsford D, Rutherford A, et al. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet. 2000;25:397–401.
Faiyaz-Ul-Haque M, Zaidi S, Al-Mureikhi M, Peltekova I, Tsui L, Teebi A. Mutations in the CHX10 gene in non-syndromic microphthalmia/anophthalmia patients from Qatar. Clin Genet. 2007;72:164–6.
Zou C, Levine EM. Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet. 2012;8:e1002924.
Ullah A, Umair M, Ahmad F, Muhammad D, Basit S, Ahmad W. A novel homozygous variant in the SMOC1 gene underlying Waardenburg anophthalmia syndrome. Ophthalmic Genet. 2017;38:335–9.
Jamshidi J, Abdollahi S, Ghaedi H, Alehabib E, Tafakhori A, Alinaghi S, et al. A novel mutation in SMOC1 and variable phenotypic expression in two patients with Waardenburg anophthalmia syndrome. Eur J Med Genet. 2017;60:578–82.
Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, et al. Correction: loss of the BMP antagonist, SMOC-1, causes ophthalmo-acromelic (Waardenburg anophthalmia) syndrome in humans and mice. PLoS Genet. 2018;14:e1007866.
Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, et al. Loss of the BMP antagonist, SMOC-1, causes ophthalmo-acromelic (Waardenburg anophthalmia) syndrome in humans and mice. PLoS Genet. 2011;7:e1002114.
Okada I, Hamanoue H, Terada K, Tohma T, Megarbane A, Chouery E, et al. SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet. 2011;88:30–41.
Suyugül Z, Seven M, Hacihanefioğlu S, Kartal A, Suyugül N, Cenani A. Anophthalmia-Waardenburg syndrome: a report of three cases. Am J Med Genet. 1996;62:391–7.
Yoshikawa Y, Yamakawa C, Shimabukuro T, Kinjo H, Fukase S, Oshiro H, et al. Progressive scoliosis associated with microphthalmia with limb anomalies: a case report. Medicine. 2023;102:e33414.
Mancini C, Zonta A, Botta G, Klobus AB, Valbonesi S, Pasini B, et al. A fetal case of microphthalmia and limb anomalies with abnormal neuronal migration associated with SMOC1 biallelic variants. Eur J Med Genet. 2019;62:103578.
Abouzeid H, Favez T, Schmid A, Agosti C, Youssef M, Marzouk I, et al. Mutations in ALDH1A3 represent a frequent cause of microphthalmia/anophthalmia in consanguineous families. Hum Mutat. 2014;35:949–53.
Aldahmesh M, Khan A, Hijazi H, Alkuraya F. Mutations in ALDH1A3 cause microphthalmia. Clin Genet. 2013;84:128–31.
Dehghani M, Tezerjani MD, Metanat Z, Mehrjardi MYV. A novel missense mutation in the ALDH13 gene causes anophthalmia in two unrelated iranian consanguineous families. IJMCM. 2017;6:131.
Liu Y, Lu Y, Liu S, Liao S. Novel compound heterozygous mutations of ALDH1A3 contribute to anophthalmia in a non-consanguineous Chinese family. Genet Mol Biol. 2017;40:430–5.
Roos L, Fang M, Dali C, Jensen H, Christoffersen N, Wu B, et al. A homozygous mutation in a consanguineous family consolidates the role of ALDH1A3 in autosomal recessive microphthalmia. Clin Genet. 2014;86:276–81.
Moretti A, Li J, Donini S, Sobol RW, Rizzi M, Garavaglia S. Crystal structure of human aldehyde dehydrogenase 1A3 complexed with NAD+ and retinoic acid. Sci Rep. 2016;6:35710.
Duester G. Towards a better vision of retinoic acid signaling during eye development. Cells. 2022;11:322.
Reis LM, Sorokina EA, Dudakova L, Moravikova J, Skalicka P, Malinka F, et al. Comprehensive phenotypic and functional analysis of dominant and recessive FOXE3 alleles in ocular developmental disorders. Hum Mol Genet. 2021;30:1591.
Wang Y, Li W, Wang Y, Huang Y. Growth inhibition of human lens epithelial cells by short hairpin RNA in transcription factor forkhead box E3 (FOXE3). Graefe’s Arch. Clin Exp Ophthalmol. 2012;250:999–1007.
Islam L, Kelberman D, Williamson L, Lewis N, Glindzicz MB, Nischal KK, et al. Functional analysis of FOXE3 mutations causing dominant and recessive ocular anterior segment disease. Hum Mutat. 2015;36:296–300.
Casey J, Kawaguchi R, Morrissey M, Sun H, McGettigan P, Nielsen JE, et al. First implication of STRA6 mutations in isolated anophthalmia, microphthalmia, and coloboma: a new dimension to the STRA6 phenotype. Hum Mutat. 2011;32:1417–26.
Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, et al. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat. 2009;30:E673–E681.
Sundaram M, Sivaprasadarao A, DeSousa M, Findlay J. The transfer of retinol from serum retinol-binding protein to cellular retinol-binding protein is mediated by a membrane receptor. J Biol Chem. 1998;273:3336–42.
Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, et al. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008;7:258–68.
Berry DC, O’Byrne SM, Vreeland AC, Blaner WS, Noy N. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Biol. 2012;32:3164.
Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, Von Lintig J. STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum Mol Genet. 2014;23:5402–17.
Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, FitzPatrick DR, Nürnberg G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–60.
Kelly M, von Lintig J. STRA6: role in cellular retinol uptake and efflux. Hepatobiliary Surg Nutr. 2015;4:229.
Chou CM, Nelson C, Tarlé SA, Pribila JT, Bardakjian T, Woods S, et al. Biochemical basis for dominant inheritance, variable penetrance, and maternal effects in RBP4 congenital eye disease. Cell. 2015;161:634–46.
Plaisancié J, Ceroni F, Holt R, Zazo Seco C, Calvas P, Chassaing N, et al. Genetics of anophthalmia and microphthalmia. Part 1: Non-syndromic anophthalmia/microphthalmia. Hum Genet. 2019;138:799–830.
Ullah M, Rehman AU, Quinodoz M, Rashid A, Cancellieri F, Munir A, et al. A comprehensive genetic landscape of inherited retinal diseases in a large Pakistani cohort. NPJ Genom Med. 2025;10:31.
Causse A, Vigouroux A, Delahaye A, Alessandri JL, Boespflug-Tanguy O, Boute-Benejean O, et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet. 2014;86:326–34.
Acknowledgements
The authors would like to thank the patients and their family members for participating in the study.
Funding
This study was supported by the Kohat University of Science & Technology by a PYP start-up grant from the National University of Singapore and the Ministry of Education, Singapore (T2EP30122-0015).
Author information
Authors and Affiliations
Contributions
Clinical data collection, sample collation, and analysis: MD, IUS, and SS; Genetic testing and data analysis: MD, XJ, NK, SX, and SS; Manuscript writing: MD and NK; Manuscript revision; SX and SS; Study supervision and coordination: NK and SS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Ethical approval and consent to participate
The study was approved by the Ethical Committee of Kohat University of Science and Technology (REF:/KUST/Ethical Committee/1050) and IRB of National University of Singapore (N-20-054E). The study was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from the participating members of the families and the parents of the minor children.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dawood, M., Ji, X., Shah, I.U. et al. Genotypic and phenotypic spectrum of anophthalmia/microphthalmia in families from Khyber Pakhtunkhwa, Pakistan. J Hum Genet 70, 565–575 (2025). https://doi.org/10.1038/s10038-025-01382-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s10038-025-01382-6