Abstract
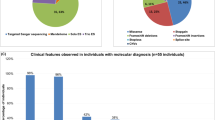
SKOR2 is a transcriptional repressor expressed in central nervous system tissues, mainly in the Purkinje cells (PCs). This is essential for the proper migration, development, and differentiation of PCs at embryonic stages, and its disruption can affect cerebellar function. SKOR2 protein has two DHD and SAND domains, which play an important role in the TGF-β signaling pathway by binding to Smad transcriptional regulators. Herein, we report nine patients from two unrelated Iranian families suffering from a distinctive combination of learning disability, facial dysmorphisms, and motor and speech impairments. Whole exome sequencing (WES) was employed to identify pathogenic variants in the probands. Sanger sequencing was conducted to confirm the mutations found in the patients, their healthy parents, and relatives. A range of bioinformatics tools was utilized to assess the impact of the identified mutations on the function and structure of the related proteins. WES identified two novel missense (c.374 G > C: p.Arg125Pro) and frameshift (c.1271_1274del: p.K424Rfs*71) mutations in exon 2 of the SKOR2 gene. After segregation and in-silico studies, autosomal recessive inheritance and pathogenic nature of the identified mutation were confirmed. In addition, the studied patients had distinct phenotypes such as clumsiness, dysarthria, and severe hypotonia compared to previous studies, which we named Skor2-related syndrome. These findings indicated a novel SKOR2-related syndrome characterized by neurodevelopmental delay and ataxia. Our findings, given the limited previous studies on the SKOR2 gene, expanded the pathogenic mutations and phenotypic spectrum of SKOR2-associated disorders, provided criteria facilitating early diagnosis and supported genetic counseling for prognosis and family planning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The identified variants of SKOR2 gene in this study are accessible on the ClinVar repository, which can be accessed via the following websites: https://www.ncbi.nlm.nih.gov/clinvar/variation/3255369/ and https://www.ncbi.nlm.nih.gov/clinvar/variation/3602131/. The raw variant call data for both identified variants have been provided as a supplementary file: https://drive.google.com/drive/folders/1XXbpkzHmXAAiNuiujtRcjgjEUanIg_dX?usp=sharing.
References
Arndt S, Poser I, Schubert T, Moser M, Bosserhoff A-K. Cloning and functional characterization of a new Ski homolog, Fussel-18, specifically expressed in neuronal tissues. Lab Investig. 2005;85:1330–41.
Arndt S, Poser I, Moser M, Bosserhoff AK. Fussel-15, a novel Ski/Sno homolog protein, antagonizes BMP signaling. Mol Cell Neurosci. 2007;34:603–11.
Minaki Y, Nakatani T, Mizuhara E, Inoue T, Ono Y. Identification of a novel transcriptional corepressor, Corl2, as a cerebellar Purkinje cell-selective marker. Gene Expr Patterns. 2008;8:418–23.
Perdomo-Sabogal A, Trakooljul N, Hadlich F, Murani E, Wimmers K, Ponsuksili S. DNA methylation landscapes from pig’s limbic structures underline regulatory mechanisms relevant for brain plasticity. Sci Rep. 2022;12:16293–305.
Fusco C, Nardella G, Morlino S, Micale L, Tragni V, Agolini E, et al. Nucleotide substitutions at the p.Gly117 and p.Thr180 mutational hot-spots of SKI alter molecular dynamics and may affect cell cycle. J Hum Genet. 2024;69:53–8.
Xu H, Wu L, Nguyen H, Mesa KR, Raghavan V, Episkopou V, et al. Arkadia-SKI/SnoN signaling differentially regulates TGF-β–induced iTreg and Th17 cell differentiation. J Exp Med. 2021;218:e20210777–94.
Sridharan R. A study of expression and function of the transcription factor Skor2 in glutamatergic neurons of the developing brainstem [Master's thesis]. Helsinki: Faculty of Biological and Environmental Sciences, University of Helsinki; 2020. 1–55.
Packi K, Matysiak J, Matuszewska E, Bręborowicz A, Matysiak J. Changes in serum protein–peptide patterns in atopic children allergic to plant storage proteins. Int J Mol Sci. 2023;24:1804–24.
Wang B, Harrison W, Overbeek PA, Zheng H. Transposon mutagenesis with coat color genotyping identifies an essential role for skor2 in sonic hedgehog signaling and cerebellum development. Development. 2011;138:4487–97.
Tecalco-Cruz AC, Ríos-López DG, Vázquez-Victorio G, Rosales-Alvarez RE, Macías-Silva M. Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease. Sig Transduct Target Ther. 2018;3:15–29.
Atamian A, Birtele M, Hosseini N, Nguyen T, Seth A, Del Dosso A, et al. Human cerebellar organoids with functional Purkinje cells. Cell Stem Cell. 2024;31:39–51.e6.
Rezk M, Pittock SJ, Kapadia RK, Knight AM, Guo Y, Gupta P, et al. Identification of SKOR2 IgG as a novel biomarker of paraneoplastic neurologic syndrome. Front. immunol. 2023;14:1243946–56.
Nakatani T, Minaki Y, Kumai M, Nitta C, Ono Y. The c-Ski family member and transcriptional regulator Corl2/Skor2 promotes early differentiation of cerebellar Purkinje cells. Dev Biol. 2014;388:68–80.
Valence S, Cochet E, Rougeot C, Garel C, Chantot-Bastaraud S, Lainey E, et al. Exome sequencing in congenital ataxia identifies two new candidate genes and highlights a pathophysiological link between some congenital ataxias and early infantile epileptic encephalopathies. Genet Med. 2019;21:553–63.
Nomura T, Khan MM, Kaul SC, Dong HD, Wadhwa R, Colmenares C, et al. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 1999;13:412–23.
Ajam-Hosseini M, Parvini F, Angaji A. A novel de novo nonsense mutation in SALL4 causing duane radial ray syndrome: a case report and expanding the phenotypic spectrum. BMC Med. Genomics. 2023;16:33–9.
Karimi AH, Karimi MR, Farnia P, Parvini F, Foroutan MA. homozygous truncating mutation in NALCN causing IHPRF1: Detailed clinical manifestations and a review of literature. Appl Clin Genet. 2020;13:151–7.
Nateghi H, Parvini F, Fahimi H. A novel homozygous missense mutation in the RPE65 gene in an Iranian family with retinitis pigmentosa. Gene Rep. 2024;35:101912.
Ramayanam NR, Manickam R, Mahalingam VT, Goh KW, Ardianto C, Ganesan P, et al. Functional and structural impact of deleterious missense single nucleotide polymorphisms in the NR3C1, CYP3A5, and TNF-α genes: an in silico analysis. Biomolecules. 2022;12:1307–24.
Gori I, George R, Purkiss AG, Strohbuecker S, Randall RA, Ogrodowicz R, et al. Mutations in ski in shprintzen–goldberg syndrome lead to attenuated tgf-β responses through ski stabilization. Elife. 2021;10:1–40.
Schepers D, Doyle AJ, Oswald G, Sparks E, Myers L, Willems PJ, et al. The SMAD-binding domain of SKI: A hotspot for de novo mutations causing Shprintzen-Goldberg syndrome. Eur J Hum Genet. 2015;23:224–8.
Chen D, Lin Q, Mian IS, Reed J, Medrano EE. The Multiple Roles of the Oncogenic Protein SKI in Human Malignant Melanoma. From Melanocytes to Melanoma: The Progression to Malignancy. 2006:211–22.
Acknowledgements
We are so grateful to patients and their respected family who kindly consented to join the study. We thank Dr. Babak Shekarchi (Radiologist, Semnan, Iran) for technical collaborations, as well. The authors also thank Semnan University and Shiraz University of Medical Sciences for their facilities and cooperation.
Author information
Authors and Affiliations
Contributions
MAFF, ZT, MAH, and FG: Experimental assays, data analysis, literature review, manuscript drafting. PN, FP, and PJ: Main idea, clinical data gathering and analysis, patient family interviews, study organization, review of clinical and laboratory data, manuscript finalization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval statement
All methods were carried out in accordance with relevant guidelines and regulations. This study had been approved by the ethics committee of the pharmaceutical sciences branch of Islamic Azad University, Tehran, Iran (ethics approval code no. IR.IAU.PS.REC.1398.209). Written informed consent was obtained from parents and father of the patients, as their legal guardians, to participate in this study. A copy of the written consent is available as requested.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farazi Fard, M.A., Tabatabaei, Z., Ajam-Hosseini, M. et al. Identification of two novel pathogenic mutations in the SKOR2 gene linked to cerebellar hypoplasia and a broad spectrum of neurodevelopmental delay in two Iranian families. J Hum Genet 70, 635–643 (2025). https://doi.org/10.1038/s10038-025-01399-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-025-01399-x
This article is cited by
-
First report of neonatal-onset glutaric aciduria type II in the Iranian population caused by a novel deleterious ETFA variant
Orphanet Journal of Rare Diseases (2025)