Abstract
Background/Objective
The evidence that maternal non-nutritive sweetener (NNS) intake during pregnancy increases childhood obesity risk is conflicting. A potential reason for this is that all prior studies examined childhood body mass index (BMI) at only one timepoint and at different ages. We examined the extent to which NNS intake during pregnancy is associated with offspring BMI z-score and body fat longitudinally from birth to 18 years.
Subjects
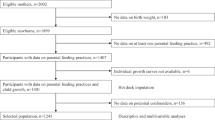
A total of 1683 children from Project Viva, a prospective pre-birth cohort, were recruited from 1999 to 2002 in Massachusetts.
Methods
We assessed maternal NNS intake in the first and second trimesters of pregnancy using a semiquantitative food frequency questionnaire. Our outcomes were offspring BMI z-score, (at birth, infancy (median 6.3 months), early childhood (3.2 years), mid-childhood (7.7 years), and early adolescence (12.9 years)), sum of skinfolds (SS), fat mass index (FMI) measured by dual x-ray absorptiometry, and BMI z-score trajectory from birth to 18 years. We adjusted models for maternal pre-pregnancy BMI, age, race/ethnicity, education, parity, pre-pregnancy physical activity, smoking, and paternal BMI and education.
Results
A total of 70% of mothers were white and pre-pregnancy BMI was 24.6 ± 5.2 kg/m2. The highest quartile of NNS intake (Q4: 0.98 ± 0.91 servings/day) was associated with higher BMI z-score in infancy (β 0.20 units; 95% CI 0.02, 0.38), early childhood (0.21; 0.05, 0.37), mid-childhood (0.21; 0.02, 0.40), and early adolescence (0.14; –0.07, 0.35) compared with Q1 intake (Q1: 0.00 ± 0.00 servings/day). Q4 was also associated with higher SS in early childhood (1.17 mm; 0.47, 1.88), mid-childhood (2.33 mm; 0.80, 3.87), and early adolescence (2.27 mm; –0.06, 4.60) and higher FMI in mid-childhood (0.26 kg/m2; –0.07, 0.59). Associations of maternal NNS intake with offspring BMI z-score became stronger with increasing age from 3 to 18 years (Pinteraction < 0.0001).
Conclusions
Maternal NNS intake during pregnancy is associated with increased childhood BMI z-score and body fat from birth to teenage years. This is relevant given the escalating obesity epidemic, and popularity of NNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Centers for Disease Control and Prevention. Obesity and overweight statistics in the United States 2015-2016. 2019. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed 21 Nov 2019.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14.
Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377:2145–53.
Buscot M-J, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimäki T, et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39:2263–70.
Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during pregnancy and childhood adiposity. Pediatrics. 2017;140:e20170031.
Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–10.
Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164:446–50.
Azad MB, Sharma AK, Souza RJ, de, Dolinsky VW, Becker AB, Mandhane PJ, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016;170:662–70.
Pepino MY, Bourne C. Non-nutritive sweeteners, energy balance, and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2011;14:391–5.
Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol. 2011;64:1430–41.
Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet Lond Engl. 1986;1:1092–3.
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–6.
Plows JF, Ponnampalam AP, Vickers MH, Reynolds CM. Artificial sweeteners during pregnancy-impact on maternal metabolic health. Reproductive Sciences. Sage Publications; 2018. p. 99A–100A.
Logan KM, Emsley RJ, Jeffries S, Andrzejewska I, Hyde MJ, Gale C, et al. Development of early adiposity in infants of mothers with gestational diabetes mellitus. Diabetes Care. 2016;39:1045–51.
Logan KM, Gale C, Hyde MJ, Santhakumaran S, Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F65–72.
Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Rawal S, Hinkle SN, et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol. 2017;46:1499–508.
Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: The EPOCH Study. J Pediatr. 2011;158:941–6.
Johnson W, Choh AC, Lee M, Towne B, Czerwinski SA, Demerath EW. Characterization of the infant bmi peak: sex differences, birth year cohort effects, association with concurrent adiposity, and heritability. Am J Hum Biol. 2013;25:378–88.
Vanderwall C, Randall Clark R, Eickhoff J, Carrel AL. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr. 2017;17:135. https://doi.org/10.1186/s12887-017-0891-z.
Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44:37–48.
Rifas-Shiman SL, Rish-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109:1004–11.
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–9.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. discussion 1127–36.
Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–62.
Project Viva | A study of health for the next generation. https://www.hms.harvard.edu/viva/data-collection-by-domain.html. Accessed 20 Nov 2020.
Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7:56.
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Oslo Nor. 1992;450(Suppl 2006):76–85.
Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–51.
Ananth CV, Schisterman EF. Confounding, causality and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167–75.
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7.
Nettleton JE, Cho NA, Klancic T, Nicolucci AC, Shearer J, Borgland SL, et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut. 2020;69:1807–17. https://doi.org/10.1136/gutjnl-2018-317505.
Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 2013;288:32475–89.
Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22:1–16.
Zhang G-H, Chen M-L, Liu S-S, Zhan Y-H, Quan Y, Qin Y-M, et al. Effects of mother’s dietary exposure to acesulfame-K in pregnancy or lactation on the adult offspring’s sweet preference. Chem Senses. 2011;36:763–70.
Liang W, Zhao Y, Lee AH. An investigation of the significance of residual confounding effect. BioMed Res Int. 2014;2014:658056. https://doi.org/10.1155/2014/658056.
Magnuson BA, Carakostas MC, Moore NH, Poulos SP, Renwick AG. Biological fate of low-calorie sweeteners. Nutr Rev. 2016;74:670–89.
Acknowledgements
We thank the participants and staff of Project Viva.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This work was supported by the US National Institutes of Health (R01 HD034568, UH3 OD023286). The authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Plows, J.F., Aris, I.M., Rifas-Shiman, S.L. et al. Associations of maternal non-nutritive sweetener intake during pregnancy with offspring body mass index and body fat from birth to adolescence. Int J Obes 46, 186–193 (2022). https://doi.org/10.1038/s41366-021-00897-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41366-021-00897-0
This article is cited by
-
Urinary excretion of low- and no-calorie sweeteners (LNCS) and associated food sources, as observed in the German cross-sectional KarMeN-study
European Journal of Nutrition (2025)