Abstract
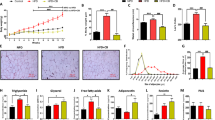
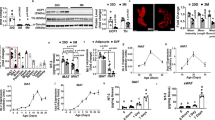
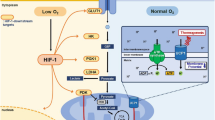
Transient receptor potential channel 5 (TRPC5) is predominantly distributed in the brain, especially in the central amygdala (CeA), which is closely associated with pain and addiction. Although mounting evidence indicates that the CeA is related to energy homeostasis, the possible regulatory effect of TRPC5 in the CeA on metabolism remains unclear. Here, we reported that the expression of TRPC5 in the CeA of mice was increased under a high-fat diet (HFD). Specifically, the deleted TRPC5 protein in the CeA of mice using adeno-associated virus resisted HFD-induced weight gain, accompanied by increased food intake. Furthermore, the energy expenditure of CeA-specific TRPC5 deletion mice (TRPC5 KO) was elevated due to augmented white adipose tissue (WAT) browning and brown adipose tissue (BAT) activity. Mechanistically, deficiency of TRPC5 in the CeA boosted nonshivering thermogenesis under cold stimulation by stimulating sympathetic nerves, as the β3-adrenoceptor (Adrb3) antagonist SR59230A blocked the effect of TRPC5 KO on this process. In summary, TRPC5 deletion in the CeA alleviated the metabolic deterioration of mice fed a HFD, and these phenotypic improvements were correlated with the increased sympathetic distribution and activity of adipose tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Mini P, Sajan Barbara C, Hansen M, Acevedo-Duncan Mark S, Kindy Denise R, Cooper Robert V. et al. Roles of hepatic atypical protein kinase C hyperactivity and hyperinsulinemia in insulin-resistant forms of obesity and type 2 diabetes mellitus. MedComm. 2021;2:3–16. https://doi.org/10.1002/mco2.54.
Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385:2400–9.
Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44.
Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Ph. 2021;320:C375–C391.
Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after fooddeprivation, cold exposure or glucoprivation. Am J Physiol Regulat Integr Comp Physiol. 2008;294:R1445–R1452.
Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature. 2019;569:229–35.
Wang P, Loh KH, Wu M, Morgan DA, Schneeberger M, Yu X, et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583:839–44.
Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104.
Castillo Armengol J, Barquissau V, Geller S, Ji H, Severi I, Venema W, et al. Hypothalamic CDK4 regulates thermogenesis by modulating sympathetic innervation of adipose tissues. Embo Rep.2020;21:e49807.
Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci. 2017;20:1384–94.
Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82.
Zhou M, Liu Z, Melin MD, Ng YH, Xu W, Sudhof TC. A central amygdala to zona incerta projection is required for acquisition and remote recall of conditioned fear memory. Nat Neurosci. 2018;21:1515–9.
Kallupi M, Wee S, Edwards S, Whitfield TJ, Oleata CS, Luu G, et al. Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychiatry. 2013;74:520–8.
Tom RL, Ahuja A, Maniates H, Freeland CM, Robinson M. Optogenetic activation of the central amygdala generates addiction-like preference for reward. Eur J Neurosci. 2019;50:2086–100.
Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci. 2010;30:14346–55.
Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–8.
Ip CK, Zhang L, Farzi A, Qi Y, Clarke I, Reed F, et al. Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab. 2019;30:111–28. e6.
Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci USA. 2019;116:6379–84.
Yuan F, Jiang H, Yin H, Jiang X, Jiao F, Chen S, et al. Activation of GCN2/ATF4 signals in amygdalar PKC-ë neurons promotes WAT browning under leucine deprivation. Nat Commun. 2020;11:2847.
Flockerzi V. An introduction on TRP channels. Springer: Berlin, Heidelberg; 2007. pp. 1–19.
Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–96.
Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24.
Sakaguchi R, Mori Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med. 2020;146:36–44.
Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104.
Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, et al. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–93.
Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, et al. TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Rep. 2017;18:583–92.
Sukumar P, Sedo A, Li J, Wilson LA, O’Regan D, Lippiat JD, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111:191–200.
Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. Plos One. 2007;2:e573.
Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–72.
Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, et al. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol. 2013;83:429–38.
Lu Z, Wei X, Sun F, Zhang H, Gao P, Pu Y, et al. Non-insulin determinant pathways maintain glucose homeostasis upon metabolic surgery. Cell Discov. 2018;4:58.
Emmett MJ, Lim H, Jager J, Richter HJ, Adlanmerini M, Peed LC, et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature. 2017;546:544–8.
Wei X, Wei X, Lu Z, Li L, Hu Y, Sun F, et al. Activation of TRPV1 channel antagonizes diabetic nephropathy through inhibiting endoplasmic reticulum-mitochondria contact in podocytes. Metabolism. 2020;105:154182.
Jian C, Fu J, Cheng X, Shen L, Ji Y, Wang X, et al. Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metab. 2020;31:892–908. e11.
Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J. 2016;40:12–21.
Betz MJ, Enerback S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol. 2018;14:77–87.
Ruiz JR, Martinez-Tellez B, Sanchez-Delgado G, Osuna-Prieto FJ, Rensen P, Boon MR. Role of human brown fat in obesity, metabolism and cardiovascular disease: strategies to turn up the heat. Prog Cardiovasc Dis. 2018;61:232–45.
Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531:647–50.
Liu T, Xu Y, Yi CX, Tong Q, Cai D. The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell. 2021;13:394–421.
Warlow SM, Naffziger EE, Berridge KC. The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat Commun. 2020;11:2716.
Kim MS, Luo S, Azad A, Campbell CE, Felix K, Cabeen RP, et al. Prefrontal cortex and amygdala subregion morphology are associated with obesity and dietary self-control in children and adolescents. Front Hum Neurosci. 2020;14:563415.
Zholos AV. TRPC5. Handb Exp Pharmacol. 2014;222:129–56.
Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–50.
Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, et al. A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev. 2011;25:2659–73.
Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, et al. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington’s disease. Brain. 2015;138:3030–47.
Bernal L, Sotelo-Hitschfeld P, Konig C, Sinica V, Wyatt A, Winter Z, et al. Odontoblast TRPC5 channels signal cold pain in teeth. Sci Adv. 2021;7:1–28.
Adke AP, Khan A, Ahn HS, Becker JJ, Wilson TD, Valdivia S, et al. Cell-type specificity of neuronal excitability and morphology in the central amygdala. eNeuro. 2021;8:1–28.
Shen B, Wong CO, Lau OC, Woo T, Bai S, Huang Y, et al. Plasma membrane mechanical stress activates TRPC5 channels. Plos One. 2015;10:e0122227.
Jonathan P, Fadok MMPT. New perspectives on central amygdala function. Curr Opin Neurobiol. 2018;49:141–7.
Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–56.
Acknowledgements
The authors thank Lutz Birnbaumer from the Laboratory of Neurobiology, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina for providing the TRPC5flox/flox mice.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81920108010, 81900761, 81873657, 81721001, and 2018YFA0800601).
Author information
Authors and Affiliations
Contributions
HM carried out all experiments in mice, acquired and analyzed data, and wrote and edited the manuscript. CH drafted and revised the manuscript. LL conceived the work and performed metabolic testing. PG designed the research protocol and revised the manuscript. ZL and YH provided methodology and analyzed data. LW performed all western blotting experiments. YZ and TC contributed to purchase of all reagents and interpreting results. YC assisted with stereotaxic brain injection experiment. HZ, GY, ZY and DL supervised the research project. ZZ conceived and directed the work, revised the manuscript and approved the final version of paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, H., He, C., Li, L. et al. TRPC5 deletion in the central amygdala antagonizes high-fat diet-induced obesity by increasing sympathetic innervation. Int J Obes 46, 1544–1555 (2022). https://doi.org/10.1038/s41366-022-01151-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-022-01151-x