Abstract
Background and objectives
Dual amylin and calcitonin receptor agonists (DACRAs) are therapeutic candidates in the treatment of obesity with beneficial effects on weight loss superior to suppression of food intake. Hence, suggesting effects on energy expenditure by possibly targeting mitochondria in metabolically active tissue.
Methods
Male rats with HFD-induced obesity received a DACRA, KBP-336, every third day for 8 weeks. Upon study end, mitochondrial respiratory capacity (MRC), - enzyme activity, - transcriptional factors, and -content were measured in perirenal (pAT) and inguinal adipose tissue. A pair-fed group was included to examine food intake-independent effects of KBP-336.
Results
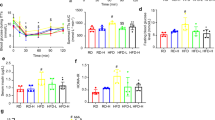
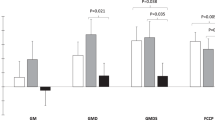
A vehicle-corrected weight loss (23.4 ± 2.8%) was achieved with KBP-336, which was not observed to the same extent with the food-restricted weight loss (12.4 ± 2.8%) (P < 0.001). Maximal coupled respiration supported by carbohydrate and lipid-linked substrates was increased after KBP-336 treatment independent of food intake in pAT (P < 0.01). Moreover, oligomycin-induced leak respiration and the activity of citrate synthase and β-hydroxyacetyl-CoA-dehydrogenase were increased with KBP-336 treatment (P < 0.05). These effects occurred without changes in mitochondrial content in pAT.
Conclusions
These findings demonstrate favorable effects of KBP-336 on MRC in adipose tissue, indicating an increased energy expenditure and capacity to utilize fatty acids. Thus, providing more mechanistic insight into the DACRA-induced weight loss.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current studies are available from the corresponding author on reasonable request.
References
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. C. Can Med Assoc J. 2020;192:E875–91.
Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine. 2023;58:101882.
Christoffersen BØ, Sanchez-Delgado G, John LM, Ryan DH, Raun K, Ravussin E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity. 2022;30:841–57.
Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol. 2020;11:617400.
Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI insight. 2020;5:e133429.
Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, Rosenstock J, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402:705–19.
Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med. 2023;389:514–26.
Lau DCW, Erichsen L, Francisco AM, Satylganova A, le Roux CW, McGowan B, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398:2160–72.
Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397:1736–48.
Andreassen KV, Larsen AT, Sonne N, Mohamed KE, Karsdal MA, Henriksen K. KBP-066A, a long-acting dual amylin and calcitonin receptor agonist, induces weight loss and improves glycemic control in obese and diabetic rats. Mol Metab. 2021;53:101282.
Larsen AT, Mohamed KE, Sonne N, Bredtoft E, Andersen F, Karsdal MA, et al. Does receptor balance matter? - Comparing the efficacies of the dual amylin and calcitonin receptor agonists cagrilintide and KBP-336 on metabolic parameters in preclinical models. Biomed Pharmacother. 2022;156:113842.
Gydesen S, Andreassen KV, Hjuler ST, Christensen JM, Karsdal MA, Henriksen K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am J Physiol Endocrinol Metab. 2016;310:E821–7.
Larsen AT, Sonne N, Andreassen KV, Gehring K, Karsdal MA, Henriksen K. The Dual Amylin and Calcitonin Receptor Agonist KBP-088 Induces Weight Loss and Improves Insulin Sensitivity Superior to Chronic Amylin Therapy. J Pharm Exp Ther. 2019;370:35–43.
Larsen AT, Sonne N, Andreassen KV, Karsdal MA, Henriksen K. The Calcitonin Receptor Plays a Major Role in Glucose Regulation as a Function of Dual Amylin and Calcitonin Receptor Agonist Therapy. J Pharm Exp Ther. 2020;374:74–83.
Hjuler ST, Gydesen S, Andreassen KV, Pedersen SLK, Hellgren LI, Karsdal MA, et al. The dual amylin- and calcitonin-receptor agonist KBP-042 increases insulin sensitivity and induces weight loss in rats with obesity. Obesity. 2016;24:1712–22.
Gydesen S, Hjuler ST, Freving Z, Andreassen KV, Sonne N, Hellgren LI, et al. A novel dual amylin and calcitonin receptor agonist, KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br J Pharm. 2017;174:591–602.
Avram VF, Merce AP, Hâncu IM, Bătrân AD, Kennedy G, Rosca MG, et al. Impairment of Mitochondrial Respiration in Metabolic Diseases: An Overview. Int J Mol Sci. 2022;23:8852.
Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165838.
Brand MD, Orr AL, Perevoshchikova IV, Quinlan CL. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 2013;169:1–8.
Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharm Toxicol. 2015;55:207–27.
Cai J, Wang F, Shao M. The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least. Endocrinol Metab. 2023;38:493–503.
Lee JH, Park A, Oh K-J, Lee SC, Kim WK, Bae K-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int J Mol Sci. 2019;20:4924.
Heinonen S, Jokinen R, Rissanen A, Pietiläinen KH. White adipose tissue mitochondrial metabolism in health and in obesity. Obes Rev J Int Assoc Study Obes. 2020;21:e12958.
Thorsø Larsen A, Karsdal MA, Henriksen K. Treatment sequencing using the dual amylin and calcitonin receptor agonist KBP-336 and semaglutide results in durable weight loss. Eur J Pharm. 2023;954:175837.
Larsen S, Danielsen JH, Søndergård SD, Søgaard D, Vigelsoe A, Dybboe R, et al. The effect of high-intensity training onmitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand J Med Sci Sports. 2015;25:59–69.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Schmidt SF, Madsen JGS, Frafjord KØ, Poulsen LL, Salö S, Boergesen M, et al. Integrative Genomics Outlines a Biphasic Glucose Response and a ChREBP-RORγ Axis Regulating Proliferation in β Cells. Cell Rep. 2016;16:2359–72.
Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008;295:E1076–83.
Kraunsøe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Støckel M, et al. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol. 2010;588:2023–32.
Thrush AB, Dent R, McPherson R, Harper M-E. Implications of mitochondrial uncoupling in skeletal muscle in the development and treatment of obesity. FEBS J. 2013;280:5015–29.
Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1855–63.
Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147:5855–64.
Wielinga PY, Löwenstein C, Muff S, Munz M, Woods SC, Lutz TA. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav. 2010;101:45–52.
Osaka T, Tsukamoto A, Koyama Y, Inoue S. Central and peripheral administration of amylin induces energy expenditure in anesthetized rats. Peptides. 2008;29:1028–35.
Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–58.
Góralska J, Śliwa A, Gruca A, Raźny U, Chojnacka M, Polus A, et al. Glucagon-like peptide-1 receptor agonist stimulates mitochondrial bioenergetics in human adipocytes. Acta Biochim Pol. 2017;64:423–9.
Coester B, Koester-Hegmann C, Lutz TA, Le Foll C. Amylin/Calcitonin Receptor-Mediated Signaling in POMC Neurons Influences Energy Balance and Locomotor Activity in Chow-Fed Male Mice. Diabetes. 2020;69:1110–25.
Li Y, Liang J, Tian X, Chen Q, Zhu L, Wang H, et al. Intermittent fasting promotes adipocyte mitochondrial fusion through Sirt3-mediated deacetylation of Mdh2. Br J Nutr. 2023;130:1473–86.
Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–8.
Childress ES, Alexopoulos SJ, Hoehn KL, Santos WL. Small Molecule Mitochondrial Uncouplers and Their Therapeutic Potential. J Med Chem. 2018;61:4641–55.
Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol J Am Coll Med Toxicol. 2011;7:205–12.
Löffler MC, Betz MJ, Blondin DP, Augustin R, Sharma AK, Tseng Y-H, et al. Challenges in tackling energy expenditure as obesity therapy: From preclinical models to clinical application. Mol Metab. 2021;51:101237.
Schöttl T, Kappler L, Fromme T, Klingenspor M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol Metab. 2015;4:631–42.
Murholm M, Dixen K, Qvortrup K, Hansen LHL, Amri E-Z, Madsen L, et al. Dynamic regulation of genes involved in mitochondrial DNA replication and transcription during mouse brown fat cell differentiation and recruitment. PLoS One. 2009;4:e8458.
Dahlman I, Forsgren M, Sjögren A, Nordström EA, Kaaman M, Näslund E, et al. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes. 2006;55:1792–9.
Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22.
Esteve Ràfols M. Adipose tissue: cell heterogeneity and functional diversity. Endocrinol y Nutr organo la Soc Esp Endocrinol y Nutr. 2014;61:100–12.
Sorensen M, Sanz A, Gómez J, Pamplona R, Portero-Otín M, Gredilla R, et al. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res. 2006;40:339–47.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Santos RX, Cardoso S, Silva S, Correia S, Carvalho C, Crisóstomo J, et al. Food deprivation promotes oxidative imbalance in rat brain. J Food Sci. 2009;74:H8–14.
Author information
Authors and Affiliations
Contributions
Emilie A. Petersen designed and performed the animal study, performed analyses, and wrote the manuscript. Ida Blom, Simone A. Melander, Mays Al-Rubai, Marina Vidotto, and Louise T. Dalgaard performed analyses. Morten A. Karsdal assisted with study design and data interpretation. Kim Henriksen, Steen Larsen, and Anna T. Larsen: assisted with study design, data interpretation, and manuscript writing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
We acknowledge the Danish Innovation Foundation and The Danish Research Foundation for funding. MAK and KH own stock in Nordic Bioscience A/S. EAP, ATL, MAK, and KH are employed by Nordic Bioscience A/S.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petersen, E.A., Blom, I., Melander, S.A. et al. DACRA induces profound weight loss, satiety control, and increased mitochondrial respiratory capacity in adipose tissue. Int J Obes 48, 1421–1429 (2024). https://doi.org/10.1038/s41366-024-01564-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01564-w