Abstract
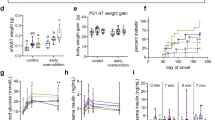
Menopause accelerates metabolic dysfunction, including (pre-)diabetes, obesity and visceral adiposity. However, the effects of endocrine vs. chronological aging in this progression are poorly understood. We hypothesized that menopause, especially in the context of middle-age, would exacerbate the metabolic effects of a high fat diet. Using young-adult and middle-aged C57BL/6J female mice, we modeled diet-induced obesity via chronic administration of high fat (HF) diet vs. control diet. We modeled peri-menopause/menopause via injections of 4-vinylcyclohexene diepoxide, which accelerates ovarian failure vs. vehicle. We performed glucose tolerance tests 2.5 and 7 months after diet onset, during the peri-menopausal and menopausal phases, respectively. Peri-menopause increased the severity of glucose intolerance and weight gain in middle-aged, HF-fed mice. Menopause increased weight gain in all mice regardless of age and diet, while chronological aging drove changes in adipose tissue distribution towards more visceral vs. subcutaneous adiposity. These data are in line with clinical data showing that post-menopausal women are more susceptible to metabolic dysfunction and suggest that greater chronological age exacerbates the effects of endocrine aging (menopause). This work highlights the importance of considering both chronological and endocrine aging in studies of metabolic health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets acquired and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11.
Christakis MK, Hasan H, De Souza LR, Shirreff L. The effect of menopause on metabolic syndrome: cross-sectional results from the Canadian Longitudinal Study on Aging. Menopause. 2020;27:999–1009.
Keller C, Larkey L, Distefano JK, Boehm-Smith E, Records K, Robillard A, et al. Perimenopausal obesity. J Womens Health. 2010;19:987–96.
Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40:2849–55.
Delamater L, Santoro N. Management of the perimenopause. Clin Obstet Gynecol. 2018;61:419–32.
Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology. 2016;31:250–7.
Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901.
Salinero AE, Anderson BM, Zuloaga KL. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. Int J Obes. 2018;42:1088–91.
Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 2015;43:776–93.
Gannon OJ, Naik JS, Riccio D, Mansour FM, Abi-Ghanem C, Salinero AE, et al. Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia. Biol sex Differ. 2023;14:34.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Ren Physiol. 2007;293:F193–9.
Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297:R587–92.
Burch KE, McCracken K, Buck DJ, Davis RL, Sloan DK, Curtis KS. Relationship between circulating metabolic hormones and their central receptors during ovariectomy-induced weight gain in rats. Front Physiol. 2021;12:800266.
Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Osorio RS, et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS ONE. 2017;12:e0185926.
Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405.
Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4:e124865. https://doi.org/10.1172/jci.insight.124865.
Razmjou S, Abdulnour J, Bastard JP, Fellahi S, Doucet É, Brochu M, et al. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: a MONET study. Menopause. 2018;25:89–97.
Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–84.
Mishra A, Brinton RD. Inflammation: bridging age, menopause and APOEε4 genotype to Alzheimer’s disease. Front Aging Neurosci. 2018;10:312.
Funding
This work was funded by an American Heart Association pre-doctoral award 908878 (AES), BrightFocus Foundation postdoctoral fellowship A2022001F (CAG), NINDS/NIA R01 NS110749 (KLZ), NIA U01 AG072464 (KLZ), Alzheimer’s Association AARG-21-849204 (KLZ).
Author information
Authors and Affiliations
Contributions
KLZ obtained funding for the experiments. KLZ, DGZ and AES designed the experiments. HLB provided consultation and training for the menopause model. AES, CAG, HV, DR, AS, RDK and OJG performed the animal work. AES analyzed the data. AES prepared the figures. KLZ and AES interpreted the results. AES prepared the manuscript. DGZ and KLZ edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salinero, A.E., Venkataganesh, H., Abi-Ghanem, C. et al. Effects of high fat diet on metabolic health vary by age of menopause onset. Int J Obes 48, 1839–1843 (2024). https://doi.org/10.1038/s41366-024-01618-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01618-z
This article is cited by
-
Loss of ovarian hormones is detrimental in early disease stages of mouse models of Alzheimer’s disease and multi-etiology dementia
Biology of Sex Differences (2025)