Abstract
Objective
To explore effective exercise types for reducing chronic inflammation in individuals with overweight and obesity (IOO) while accounting for confounders.
Methods
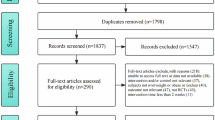
A systematic search for RCTs in English between January 2000 and August 2023 was conducted to evaluating exercise effects on inflammatory biomarkers in IOO. A network meta-analysis conducted.
Results
A total of 123 RCTs were analyzed. Different exercise type yielded distinct effects on various inflammatory biomarkers. Specifically, aerobic exercise combined with resistance training (COM) and aerobic exercise (AE) were the most effective for improving leptin levels. AE exhibited the greatest effectiveness in reducing CRP and increasing adiponectin. High-intensity interval training (HIIT) was identified as the most effective exercise modality for ameliorating IL-6, TNF-α, and IL-10. Resistance training (RT) had the least effect compared to other exercise types. Meta regression and subgroup analyses revealed that high-intensity AE demonstrated a greater effect size compared to moderate-intensity AE. The impact of AE on IL-10 was positively associated with both the training period and the age of participants. Positive correlations were observed between reductions in body fat and the effect sizes of CRP, TNF-α, and IL-10. Gender influenced AE effects on IL-6 and TNF-α, with females responding better.
Conclusion
This study highlights the potential of exercise in alleviating the inflammatory status in IOO, with different exercise types showing various effects on specific inflammatory biomarkers. The intensity and duration of exercise had a dose-response relationship with intervention effectiveness. Changes in body composition correlated with the effectiveness of the intervention. COM, AE, and HIIT are recommended exercise approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Martin-Rodriguez E, Guillen-Grima F, Martí A, Brugos-Larumbe A. Comorbidity associated with obesity in a large population: the APNA study. Obes Res Clin Pr. 2015;9:435–47.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Sikaris KA. The clinical biochemistry of obesity. Clin Biochem Rev. 2004;25:165–81.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pr. 2014;105:141–50.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34.
Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–62.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Trayhurn P. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2022;127:161–4.
Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–44.
Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–15.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50.
Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 2021;12:585887.
Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36.
Aycan Z, Berberoğlu M, Ocal G, Evliyaoglu O, Adiyaman P, Deda G, et al. Relationship between plasma leptin, insulin and tumor necrosis factor alpha in obese children. J Pediatr Endocrinol Metab. 2005;18:275–84.
Zocchi M, Della Porta M, Lombardoni F, Scrimieri R, Zuccotti GV, Maier JA, et al. A potential interplay between HDLs and adiponectin in promoting endothelial dysfunction in obesity. Biomedicines. 2022;10:1344.
Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: role of superoxide. Diabetes. 2002;51:1931–7.
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765.
Shaikh SR, Haas KM, Beck MA, Teague H. The effects of diet-induced obesity on B cell function. Clin Exp Immunol. 2015;179:90–9.
Man K, Kallies A, Vasanthakumar A. Resident and migratory adipose immune cells control systemic metabolism and thermogenesis. Cell Mol Immunol. 2022;19:421–31.
Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: past, present and future. Crit Rev Clin Lab Sci. 2015;52:180–90.
Jaleel A, Aheed B, Jaleel S, Majeed R, Zuberi A, Khan S, et al. Association of adipokines with obesity in children and adolescents. Biomark Med. 2013;7:731–5.
Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones. 2012;11:8–20.
Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med. 2010;44:620–30.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83.
Zhang G, Yu P, Liu X. Swim training attenuates inflammation and improves insulin sensitivity in mice fed with a high-fat diet. Int J Endocrinol. 2017;2017:5940732.
Martins FM, de Paula Souza A, Nunes PRP, Michelin MA, Murta EFC, Resende E, et al. High-intensity body weight training is comparable to combined training in changes in muscle mass, physical performance, inflammatory markers and metabolic health in postmenopausal women at high risk for type 2 diabetes mellitus: a randomized controlled clinical trial. Exp Gerontol. 2018;107:108–15.
Barry JC, Simtchouk S, Durrer C, Jung ME, Mui AL, Little JP. Short-term exercise training reduces anti-inflammatory action of interleukin-10 in adults with obesity. Cytokine. 2018;111:460–9.
Fedewa MV, Hathaway ED, Higgins S, Forehand RL, Schmidt MD, Evans EM. Moderate, but not vigorous, intensity exercise training reduces C-reactive protein. Acta Cardiol. 2018;73:283–90.
Gerosa-Neto J, Antunes BM, Campos EZ, Rodrigues J, Ferrari GD, Rosa Neto JC, et al. Impact of long-term high-intensity interval and moderate-intensity continuous training on subclinical inflammation in overweight/obese adults. J Exerc Rehabil. 2016;12:575–80.
Vella CA, Taylor K, Drummer D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur J Sport Sci. 2017;17:1203–11.
Sirico F, Bianco A, D’Alicandro G, Castaldo C, Montagnani S, Spera R, et al. Effects of physical exercise on adiponectin, leptin, and inflammatory markers in childhood obesity: systematic review and meta-analysis. Child Obes. 2018;14:207–17.
Wang S, Zhou H, Zhao C, He H. Effect of exercise training on body composition and inflammatory cytokine levels in overweight and obese individuals: a systematic review and network meta-analysis. Front Immunol. 2022;13:921085.
Lee J. Influences of exercise interventions on overweight and obesity in children and adolescents. Public Health Nurs. 2021;38:502–16.
Makarewicz A, Jamka M, Geltz J, Smidowicz A, Kokot M, Kaczmarek N, et al. Comparison of the effect of endurance, strength, and endurance-strength training on inflammatory markers and adipokines levels in overweight and obese adults: systematic review and meta-analysis of randomised trials. Healthcare. 2022;10:1098.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions, Version 6.3 Cochrane. Hoboken, NJ: Wiley; 2022. Available from: www.training.cochrane.org/handbook.
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:e76654.
Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6:79.
O’Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. 2021;22:e13137.
Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4.
Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100.
Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8:1031–63.
Yu N, Ruan Y, Gao X, Sun J. Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Horm Metab Res. 2017;49:164–73.
Del Rosso S, Baraquet ML, Barale A, Defagó MD, Tortosa F, Perovic NR, et al. Long-term effects of different exercise training modes on cytokines and adipokines in individuals with overweight/obesity and cardiometabolic diseases: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Obes Rev. 2023;24:e13564.
Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61.
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12.
Ferrante AW Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–14.
Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409.
Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31.
Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442–54.
Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51:670–6.
Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–21.
Tenório TRS, Balagopal PB, Andersen LB, Ritti-Dias RM, Hill JO, Lofrano-Prado MC, et al. Effect of low- versus high-intensity exercise training on biomarkers of inflammation and endothelial dysfunction in adolescents with obesity: a 6-month randomized exercise intervention study. Pediatr Exerc Sci. 2018;30:96–105.
L ZS, X J. Effects of exercise intervention on adipocyte cytokines such as leptin in obese bodies. Prog Physiol Sci. 2023;54:141–7.
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–49.
Virdis A, Colucci R, Bernardini N, Blandizzi C, Taddei S, Masi S. Microvascular endothelial dysfunction in human obesity: role of TNF-α. J Clin Endocrinol Metab. 2019;104:341–8.
Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–8.
You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43:243–56.
Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–78.
Chen X, Sun X, Gao D, Qiu D, He H. A meta-analysis of the effects of aerobic exercise on the basal level of endothelial progenitor cells in middle-aged and older adults. J Aging Phys Act. 2022;30:610–8.
Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812.
De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, et al. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007;293:E713–25.
Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–33.
Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62.
Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–7.
Szostak J, Laurant P. The forgotten face of regular physical exercise: a ‘natural’ anti-atherogenic activity. Clin Sci. 2011;121:91–106.
Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–61.
Liu M, Lin X, Wang X. Decrease in serum chemerin through aerobic exercise plus dieting and its association with mitigation of cardio-metabolic risk in obese female adolescents. J Pediatr Endocrinol Metab. 2018;31:127–35.
Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma. a randomized controlled trial. Am J Respir Crit Care Med. 2017;195:32–42.
Author information
Authors and Affiliations
Contributions
This systematic review and network meta-analysis was designed by CFC and XKC. CFC and DZ conducted literature searches and selected articles for inclusion. YLS extracted data checked the extracted data. YWY contributed to data curation and analysis. CFC and DZ wrote the manuscript. XKC revised the manuscript, and YE polished the language. All author read and approved the final version. XKC responsible for supervision, review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Zhang, D., Ye, M. et al. Effects of various exercise types on inflammatory response in individuals with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Int J Obes 49, 214–225 (2025). https://doi.org/10.1038/s41366-024-01649-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01649-6
This article is cited by
-
Comparative effects of different exercise types on inflammatory markers in type 2 diabetes mellitus patients with overweight and obesity: a systematic review and network meta-analysis
BMC Endocrine Disorders (2026)
-
Optimal exercise modalities and dose selection to reduce leptin levels in overweight or obese individuals: a network meta-analysis and dose–response relationship study
BMC Sports Science, Medicine and Rehabilitation (2025)
-
Effects of exercise alone or combined with dietary restriction on leptin and adiponectin in overweight or obese individuals: a network meta-analysis
BMC Sports Science, Medicine and Rehabilitation (2025)
-
The intensity of exercise and inflammation markers in women with overweight & obesity: a systematic review and network meta-analysis
International Journal of Obesity (2025)
-
Impact of exercise on immune cell infiltration in muscle tissue: implications for muscle repair and chronic disease
Clinical and Experimental Medicine (2025)