Abstract
Background
Understanding how obesity impacts human mammary adipose tissue (MAT) biology is crucial for deciphering its role in mammary epithelium during both physiological and pathophysiological processes, including breast cancer. Hypertrophic mammary adipocytes and Crown-Like Structures are present in MAT of patients with obesity but whether these changes initiate a fibro-inflammatory response at the tissue level remains insufficiently explored.
Objective
We investigated the markers of adipose tissue dysfunction (immune cell infiltration, secretion pattern and fibrosis) in tumor-free MAT of patients with obesity versus patients who are lean.
Methods
Tumor-free MAT were obtained from 96 women with (n = 43) or without (n = 53) obesity who underwent mastectomy for breast cancer risk reduction or treatment. Immune and non-immune cell infiltration were determined using flow cytometry. Bulk transcriptomic was used to characterize the phenotype of CD206+ macrophages whose infiltration is increased in patients with obesity. Conditioned-medium were prepared from MAT to characterize their secretome and dose adipokines and cytokines by ELISA assay. The extra-cellular matrix (ECM) deposition was evaluated by Masson trichrome staining on cross-stained sections, 3D imaging of red picrosirius-stained tissues and measure of hydroxyproline content.
Results
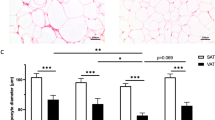
We observed an increase of CD206+/HLA-DR+ macrophages in the stromal vascular fraction of MAT from patients with obesity compared to patients who are lean. Other immune cell infiltration and endothelial or adipose progenitor cell numbers were similar between groups. Bulk transcriptomics on CD206+ macrophages revealed a significant decrease in ECM component expression and processing in obesity. In addition, no heightened secretion of pro-inflammatory cytokines, TGF-β1 or MCP-1 was observed in the samples from patients with obesity. ECM characterization revealed an absence of fibrosis, with MAT of patients with obesity showing even a slightly reduced collagen secretion and deposition compared with their lean counterparts.
Conclusions
Obesity is not associated with inflammation nor fibrosis in MAT, highlighting its unique behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Neville MC, Medina D, Monks J, Hovey RC. The mammary fat pad. J Mammary Gland Biol Neoplasia. 1998;3:109–16.
Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018;27:68–83.
Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. 2010;15:279–90.
Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–58.
Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 2018;28:282–8.e3.
Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98.
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–14.
Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1:611–21.
Harris BHL, Macaulay VM, Harris DA, Klenerman P, Karpe F, Lord SR, et al. Obesity: a perfect storm for carcinogenesis. Cancer Metastasis Rev. 2022;41:491–515.
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65.
Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2:e87489.
Wang YY, Lehuédé C, Laurent V, Dirat B, Dauvillier S, Bochet L, et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142–51.
Attané C, Muller C. Drilling for oil: tumor-surrounding adipocytes fueling cancer. Trends Cancer. 2020;6:593–604.
Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990–4000.
Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44.
Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 2017;25:673–85.
Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021–9.
Vaysse C, Lømo J, Garred Ø, Fjeldheim F, Lofteroed T, Schlichting E, et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer. 2017;3:19.
Laforest S, Ennour-Idrissi K, Ouellette G, Gauthier MF, Michaud A, Durocher F, et al. Associations between markers of mammary adipose tissue dysfunction and breast cancer prognostic factors. Int J Obes. 2005;45:195–205.
Springer NL, Iyengar NM, Bareja R, Verma A, Jochelson MS, Giri DD, et al. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am J Pathol. 2019;189:2019–35.
Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–12.
Coradini D, Gambazza S, Oriana S, Ambrogi F. Gene expression profile of normal breast tissue and body mass index. Breast Cancer. 2021;28:488–95.
de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26.
Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130.
Colleluori G, Perugini J, Barbatelli G, Cinti S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev Endocr Metab Disord. 2021;22:241–55.
Roumiguié M, Estève D, Manceau C, Toulet A, Gilleron J, Belles C, et al. Periprostatic adipose tissue displays a chronic hypoxic state that limits its expandability. Am J Pathol. 2022;192:926–42.
Estève D, Boulet N, Belles C, Zakaroff-Girard A, Decaunes P, Briot A, et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat Commun. 2019;10:2549.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Ben-Ari Fuchs S, Lieder I, Stelzer G, Mazor Y, Buzhor E, Kaplan S, et al. GeneAnalytics: an integrative gene set analysis tool for next generation sequencing, RNAseq and microarray data. Omics J Integr Biol. 2016;20:139–51.
Ayoub I, Dauvilliers Y, Barateau L, Vermeulen T, Mouton-Barbosa E, Marcellin M, et al. Cerebrospinal fluid proteomics in recent-onset Narcolepsy type 1 reveals activation of the complement system. Front Immunol. 2023;14:1108682.
Duguet F, Locard-Paulet M, Marcellin M, Chaoui K, Bernard I, Andreoletti O, et al. Proteomic analysis of regulatory T cells reveals the importance of Themis1 in the control of their suppressive function. Mol Cell Proteom. 2017;16:1416–32.
Bouyssié D, Hesse AM, Mouton-Barbosa E, Rompais M, Macron C, Carapito C, et al. Proline: an efficient and user-friendly software suite for large-scale proteomics. Bioinformatics. 2020;36:3148–55.
Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–15.
Clement E, Lazar I, Attané C, Carrié L, Dauvillier S, Ducoux-Petit M, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020;39:e102525.
Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clément K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest. 2019;129:4032–40.
Marcos-Garcés V, Harvat M, Molina Aguilar P, Ferrández Izquierdo A, Ruiz-Saurí A. Comparative measurement of collagen bundle orientation by Fourier analysis and semiquantitative evaluation: reliability and agreement in Masson’s trichrome, Picrosirius red and confocal microscopy techniques. J Microsc. 2017;267:130–42.
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91.
Xia T, Shen Z, Cai J, Pan M, Sun C. ColXV aggravates adipocyte apoptosis by facilitating abnormal extracellular matrix remodeling in mice. Int J Mol Sci. 2020;21:959.
Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–7.
Rebeaud M, Bouche C, Dauvillier S, Attané C, Arellano C, Vaysse C, et al. A novel 3D culture model for human primary mammary adipocytes to study their metabolic crosstalk with breast cancer in lean and obese conditions. Sci Rep. 2023;13:4707.
Nguyen HL, Geukens T, Maetens M, Aparicio S, Bassez A, Borg A, et al. Obesity-associated changes in molecular biology of primary breast cancer. Nat Commun. 2023;14:4418.
Acknowledgements
This work benefited from the Toulouse Réseau Imagerie (TRI)-RIO Optical Imaging Platform at the Institute of Pharmacology and Structural Biology (Genotoul, Toulouse, France). We thank Mathilde Lacombe (IPBS, Toulouse) for her writing assistance. We thank Jessica Tran for her contribution in the hydroxyproline quantification experiments.
Funding
This study was funded by the Ligue Contre le Cancer (Comité Midi-Pyrénées), and by the Institut National Contre le Cancer (Programme d’actions intégrées de recherche (PAIR)—Obésités et Cancers, INCa_18711). This study was funded in part by the French Ministry of Research with the Investissement d’Avenir Infrastructures Nationales en Biologie et Santé program (ProFI, Proteomics French Infrastructure project, ANR-10-INBS-08) (to OBS). LO received a master fellowship (Program “Espoirs de la recherche”) from the Fondation pour la Recherche Médicale (FRM). MR received a PhD fellowship from the Ligue Nationale contre le Cancer and from the Fondation pour la Recherche Médicale (FRM). CA received a master fellowship from the Association pour la Recherche sur le Cancer (ARC).
Author information
Authors and Affiliations
Contributions
FF, MR and CB performed and analyzed most of the experiments presented in the manuscript and prepared the figures. SD and CA performed and prepared the figures of the imaging experiments (3D picrosirius staining). LO and RD performed the flow cytometry experiments with the help of JF under the supervision of AB. LO prepared the samples for proteomic analysis, FF, MDP and EMB performed the analysis of the proteomic data under the supervision of OBS. RN and FF performed the analysis of the RNA-seq. CF performed the Trichrome Masson staining which was analyzed by CV and DE. CA, CB and CV supervised the collection of human mammary adipose tissue samples and MM participated to tissue processing. AB, CV, FF, and CM supervised the study and wrote the manuscript. FF and MR performed the experiments required for the revision process. All the authors reviewed the manuscript. All the authors agreed with the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fallone, F., Rebeaud, M., Bouche, C. et al. Lack of fibro-inflammatory response in human mammary adipose tissue in obesity. Int J Obes 49, 809–818 (2025). https://doi.org/10.1038/s41366-024-01705-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01705-1