Abstract
Background
Obesity plays a crucial role in the development of metabolic disorders including diabetes, coronary and renal diseases. There are several factors involved in the pathology of obesity, including chronic inflammation and exposure to environmental contaminants. Recently, the cholinergic co-hydrolyzing enzyme BChE has been associated with clinical conditions such as diabetes and obesity. This study aims to investigate the levels of BChE and inflammatory markers in the serum, as well as the association between two specific BCHE gene variants (rs1803274 and rs3495) and the risk of obesity in the Pakistani population.
Methods
The study recruited 350 people with obesity and 200 volunteers with no obesity. Proinflammatory cytokines (TNF-α, IL-6, and IL-1β) levels were quantified using ELISA kits, while the analysis of BCHE gene SNPs rs1803274 (K-variant) and rs3495 was conducted using the tetra-primer amplification refractory mutation-PCR (tetra-ARM-PCR) and PCR-restriction fragment length polymorphism (RFLP) methods, respectively. Additionally, clinico-pathological parameters HDL, LDL, BMI, Homa-IR, insulin, glucose, blood pressure was also assessed in subjects of current study.
Results
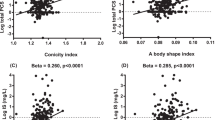
Results showed significantly higher levels of BChE, TNF-α, IL-1β, and IL-6 in the obesity group compared to the group without obesity. Furthermore, the obesity group exhibited higher blood pressure and LDL levels, as well as lower HDL levels when compared to group without obesity. Logistic regression analysis revealed a relationship between obesity and higher BChE activity, blood pressure, LDL, and lower HDL levels. The study also found a statistically significant association between the BCHE gene SNPs rs1803274 (K-variant) and rs3495 and the risk of obesity (OR = 2.01; CI = 1.21–3.33; p = 0.0063; OR = 1.80; CI = 1.09–2.96, respectively).
Conclusions
In conclusion, the study suggests that BChE and inflammatory cytokines play a significant role in the development and pathogenesis of obesity and can also act as good diagnostic biomarkers for obesity and its related metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data will be made available on request.
References
Phelps NH, Singleton RK, Zhou B, Heap RA, Mishra A, Bennett JE, et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. The Lancet. 2024;403:1027–50.
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10.
Bray GA. Pathophysiology of obesity. American J Clin Nutr. 1992;55:488S–94S.
Han Y, Ma Y, Liu Y, Zhao Z, Zhen S, Yang X, et al. Plasma cholinesterase is associated with Chinese adolescent overweight or obesity and metabolic syndrome prediction. Diabetes Metab Syndr Obes. 2019;14:685–702.
Nicolaidis S. Environment and obesity. Metabolism. 2019;100:153942.
Chen YC, Chen PC, Hsieh WS, Portnov BA, Chen YA. Yungling LL. Environmental factors associated with overweight and obesity in taiwanese children. Paediatr Perinat Epidemiol. 2012;26:561–71.
Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51.
Zaitlen N, Kraft P, Patterson N, Pasaniuc B, Bhatia G, Pollack S, et al. Using extended genealogy to estimate components of heritability for 23 quantitative and dichotomous traits. PLoS Genet. 2013;9:e1003520.
Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6:223–36.
Nettore IC, Franchini F, Palatucci G, Macchia PE, Ungaro P. Epigenetic mechanisms of endocrine-disrupting chemicals in obesity. Biomedicines. 2021;9:1716.
Pezzementi L, Nachon F, Chatonnet A. Evolution of acetylcholinesterase and butyrylcholinesterase in the vertebrates: an atypical butyrylcholinesterase from the Medaka Oryzias latipes. PLoS One. 2011;6:e17396.
Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–8.
Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34.
Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–96.
Frouni I, Kwan C, Belliveau S, Huot P. Cognition, and serotonin in Parkinson’s disease. Prog Brain Res. 2022;269:373–403.
Reale M, Costantini E. Cholinergic modulation of the immune system in neuroinflammatory diseases. Diseases. 2021;9:29.
Bono GF, Simão-Silva DP, Batistela MS, Josviak ND, Dias PF, Nascimento GA, et al. Butyrylcholinesterase: K variant, plasma activity, molecular forms and rivastigmine treatment in Alzheimer’s disease in a Southern Brazilian population. Neurochem Int. 2015;81:57–62.
Babaoglu MO, Ocal T, Bayar B, Kayaalp SO, Bozkurt A. Frequency and enzyme activity of the butyrylcholinesterase K-variant in a Turkish population. Eur J Clin Pharmacol. 2004;59:875–7.
Mbah Ntepe LJ, Habib R, Judith Laure N, Raza S, Nepovimova E. Kamil K, et. al. Oxidative Stress and Analysis of Selected SNPs of ACHE (rs 2571598), BCHE (rs 3495), CAT (rs 7943316), SIRT1 (rs 10823108), GSTP1 (rs 1695), and Gene GSTM1, GSTT1 in Chronic Organophosphates Exposed Groups from Cameroon and Pakistan. Int J Mol Sci. 2020;21:6432.
Bartels CF, Van der Spek AF, La Du BN. Two polymorphisms in the non-coding regions of the BCHE gene. Nucleic Acids Res. 1990;18:6171.
Furtado-Alle L, Tureck LV, de Oliveira CS, Hortega JV, Souza RL. Butyrylcholinesterase and lipid metabolism: Possible dual role in metabolic disorders. Chem Biol Interact. 2023;383:110680.
Chaves TJ, Leite N, Milano GE, Milano GE, Souza RL, Chautard-Freire-Maia EA, et al. 116A and K BCHE gene variants associated with obesity and hypertriglyceridemia in adolescents from Southern Brazil. Chem Biol Interact. 2013;203:341–3.
Ellman GL, Courtney KD, Andres JrV, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.
Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Lahiri DK, Nurnberger JrJI. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444.
Patočka J, Kuča K, Jun D. Acetylcholinesterase and butyrylcholinesterase–important enzymes of human body. Acta Medica. 2004;47:215–28.
Pohanka M. Butyrylcholinesterase as a biochemical marker. Bratisl Lek Listy. 2013;114:726–34.
Gazzi EN, Sorodoc V, Jaba IM, Lionte C, Bologa C, Lupusoru CE, et al. Profile of adult acute cholinesterase inhibitors substances poisoning–a 30-year analysis. Open Medicine. 2015;10:278–84.
Ogunkeye OO, Roluga AI. Serum cholinesterase activity helps to distinguish between liver disease and non-liver disease aberration in liver function tests. Pathophysiology. 2006;13:91–3.
Hamouda AF, Khardali IA, Attafi IM, Oraiby ME, Attafi MA, Muyidi AM, et al. Study the Relation Between Acetylcholinesterase and Obesity in University Students. Int J of Nutr Food Sci. 2019;8:46–51.
Rao AA, Reddy CS, Sridhar GR, Annapurna A, Hanuman T, Prameela M, et al. Enhanced butyrylcholinesterase activity may be the common link in triggering low-grade systemic inflammation and decrease in cognitive function in diabetes mellitus and Alzheimer’s disease. Curr Nutr Food Sci. 2008;4:213–6.
Tangvarasittichai S, Pongthaisong S, Meemark S, Tangvarasittichai O. Abdominal obesity associated with elevated serum butyrylcholinesterase activity, insulin resistance and reduced high density lipoprotein-cholesterol levels. Indian J Clin Biochem. 2015;30:275–80.
Boberg DR, Furtado-Alle L, Souza RL, Chautard-Freire-Maia EA. Molecular forms of butyrylcholinesterase and obesity. Genet Mol Biol. 2010;33:452–4.
De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005.
Valle AM, Radić Z, Rana BK, Whitfield JB, O’Connor DT, Martin NG, et al. The cholinesterases: analysis by pharmacogenomics in man. Chem Biol Interact. 2008;175:343–5.
Ha ZY, Mathew S, Yeong KY. Butyrylcholinesterase: a multifaceted pharmacological target and tool. Curr Protein Pept Sci. 2020;21:99–109.
Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: the cholinergic anti-inflammatory pathway. J Endotoxin Res. 2003;9:409–13.
Frühbeck G. The adipose tissue as a source of vasoactive factors. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:197–208.
Caër C, Rouault C, Le Roy T, Poitou C, Aron-Wisnewsky J, Torcivia A, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep. 2017;7:3000.
Munir S, Habib R, Awan S, Bibi N, Tanveer A, Batool S, et al. Biochemical Analysis and Association of Butyrylcholinesterase SNPs rs3495 and rs1803274 with Substance Abuse Disorder. J Mol Neurosci. 2019;67:445–55.
Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther. 2015;148:34–46.
Shields KA, Lewis J. The Identification of Butyrylcholinesterase (BCHE) Polymorphisms in a Small Australian Defence Force Cohort. Human Protection and Performance Division, Defence Science and Technology Organisation; 2011.
Aeinehband S, Lindblom RP, Al Nimer F, Vijayaraghavan S, Sandholm K, Khademi M, et al. Complement component C3 and butyrylcholinesterase activity are associated with neurodegeneration and clinical disability in multiple sclerosis. PLoS One. 2015;10:e0122048.
Pleva L, Kovarova P, Faldynova L, Plevova P, Hilscherova S, Zapletalova J, et al. The rs1803274 polymorphism of the BCHE gene is associated with an increased risk of coronary in-stent restenosis. BMC Cardiovasc Disord. 2015;15:1–9.
Habieb MS, Elhelbawy NG, Alhanafy AM, Elhelbawy MG, Alkelany AS, Wahb AM. Study of the potential association of the BCHE rs1803274 genetic polymorphism and serum level of its protein with breast cancer. Meta Gene. 2021;29:100913.
Negrão AB, Pereira AC, Guindalini C, Santos HC, Messas GP, Laranjeira R, et al. Butyrylcholinesterase genetic variants: association with cocaine dependence and related phenotypes. PLoS One. 2013;8:e80505.
Oliveira JD, Tureck LV, Santos WD, Saliba LF, Schenknecht CS, Scaraboto D, et al. Effect of BCHE single nucleotide polymorphisms on lipid metabolism markers in women. Genet Mol Biol. 2017;40:408–14.
Lima JK, Leite N, Turek LV, Souza RL, da Silva Timossi L, Osiecki AC, et al. 1914G variant of BCHE gene associated with enzyme activity, obesity and triglyceride levels. Gene. 2013;532:24–6.
Kálmán J, Juhász A, Rakonczay Z, Ábrahám G, Zana M, Boda K, et al. Increased serum butyrylcholinesterase activity in type IIb hyperlipidaemic patients. Life Sci. 2004;75:1195–204.
Benyamin B, Middelberg RP, Lind PA, Valle AM, Gordon S, Nyholt DR, et al. GWAS of butyrylcholinesterase activity identifies four novel loci, independent effects within BCHE and secondary associations with metabolic risk factors. Hum Mol Genet. 2011;20:4504–14.
Askalsky P, Kalapatapu RK, Foltin RW, Comer SD. Butyrylcholinesterase levels and subjective effects of smoked cocaine in healthy cocaine users. Am J Drug Alcohol Abuse. 2015;41:161–5.
Acknowledgements
The authors are thankful to the participating individuals in the study. Thanks to the Department of Biosciences, COMSATS University Islamabad, Pakistan for providing facilities to conduct experiments. This work was supported by Excellence project PrF UHK 2208/2024-2025 and MHCZ - DRO (UHHK, 00179906), Long term development plan UHK.
Author information
Authors and Affiliations
Contributions
Amna Amir: conceptualization, experiment, data analysis and writing first original draft. Sabir Hussain: supervising genetics part, formal data analysis and review draft. Syed Tahir Abbas Shah: edit and review the draft, data curation. Rabia Habib: data acquisition, review, and editing. Zahid Muneer: data acquisition, edit and review the draft. Eugenie Nepovimova: Resources, formal data analysis, review of draft. Kamil Kuca: conceptualization, resources, and final approval of draft. Syed Muhammad Nurulain: Supervisor, project administration, study design and final approval of draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Ethical Review Board of the Department of Biosciences, COMSATS University Islamabad (CUI/BIO/ERB/2021/52).
Consent to participate
Consent for the participation in study was obtained from volunteers. A proforma with the information relating to obesity category, age, sex, work practices, dietary habits and other demographic aspects was designed and filled for each participating individual with their consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amir, A., Hussain, S., Shah, S.T.A. et al. Association of BCHE gene SNP rs1803274 (K-variant) and rs3495 with obesity in Pakistani population group. Int J Obes 49, 881–887 (2025). https://doi.org/10.1038/s41366-025-01715-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01715-7