Abstract
Background
The implication of body composition and energy metabolism in the control of human energy intake (EI) has been well described in adults, remaining however unexplored in adolescents with obesity. The aim of this study was to question the role of body composition, energy expenditure (EE) and substrate metabolism in the control of EI of adolescents with obesity.
Methods
Ad libitum 24-h EI, body composition (Dual X-ray absorptiometry), Resting Metabolic Rate (RMR, indirect calorimeter) where measured and Total EE obtained during a 36-h stay in metabolic chambers in 26 adolescents (14.1 ± 1.5 years; 14 girls) with severe obesity.
Results
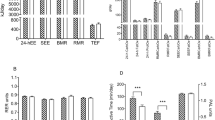
The mean body weight and Body Mass index were 92.2 ± 17.2 kg and 33.98 ± 4.14 kg.m-2 respectively. 24-h EI was positively correlated with body weight (rho = 0.597, p = 0.014), Fat Free Mass (FFM) kg (rho = 0.576, p = 0.019), 24-h Total EE (TEE (rho =0.675, p < 0.001)), RMR (rho =0.632, p = 0.005), 24-h Carbohydrate (CHO) oxidation rates (rho = 0.716, p < 0.001), and urinary nitrogen excretion (rho =0.28, p < 0.001). According to the path analysis FFM (kg) but not Fat Mass (FM) (kg) was positively correlated with RMR, with direct effects of 0.87 (p < 0.001) and 0.027 (p = 0.74) respectively. The effect of FFM on 24-h EI was mediated by RMR (96% of the effect), while the effect of FM on 24-h EI was also mediated by RMR (67% of the effect).
Conclusions
The present study provides the first evidence regarding the role of RMR as a main tonic signal of appetite control mediating the effect of body composition and mainly FFM (over FM) on daily EI in adolescents with obesity. It also suggests for the first-time relationships between 24-h CHO and protein oxidation and daily EI in this population.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data might be available upon request.
References
Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–14.
Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–64.
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95.
Woods SC, Ramsay DS. Food intake, metabolism and homeostasis. Physiol Behav. 2011;104:4–7.
Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech. 2012;5:608–13.
Blundell JE, Gibbons C, Beaulieu K, Casanova N, Duarte C, Finlayson G, et al. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol Behav. 2020;219:112846.
Hopkins M, Gibbons C, Blundell J. Fat-free mass and resting metabolic rate are determinants of energy intake: implications for a theory of appetite control. Philos Trans R Soc Lond B Biol Sci. 2023;378:20220213.
Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin Sci Lond Engl 1979. 2016;130:1615–28.
Blundell JE, Caudwell P, Gibbons C, Hopkins M, Näslund E, King NA, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–9.
Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Näslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97:7–14.
Grannell A, Al-Najim W, Mangan A, Kapoor N, Martin WP, Murphy JC, et al. Fat free mass is positively associated with hunger and energy intake at extremes of obesity. Appetite. 2019;143:104444.
McNeil J, Lamothe G, Cameron JD, Riou MÈ, Cadieux S, Lafrenière J, et al. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake? Am J Clin Nutr. 2017;106:1206–12.
Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes 2005. 2014;38:243–51.
Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int J Obes 2005. 2016;40:312–8.
Hopkins M, Finlayson G, Duarte C, Gibbons C, Johnstone AM, Whybrow S, et al. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. Int J Obes 2005. 2019;43:233–42.
Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab. 2015;100:3011.
Wells JC, Davies PS, Hopkins M, Blundell JE. The « drive to eat » hypothesis: energy expenditure and fat-free mass but not adiposity are associated with milk intake and energy intake in 12 week infants. Am J Clin Nutr. 2021;114:505–14.
Hopkins M, Casanova N, Finlayson G, Stubbs RJ, Blundell JE. Fat-free mass and total daily energy expenditure estimated using doubly labeled water predict energy intake in a large sample of community-dwelling older adults. J Nutr. 2022;152:971–80.
Cameron JD, Sigal RJ, Kenny GP, Alberga AS, Prud’homme D, Phillips P, et al. Body composition and energy intake - skeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Appl Physiol Nutr Metab Physiol Appl Nutr Metab Juin. 2016;41:611–7.
Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–32.
Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord. 1996;20:393–405.
Isacco L, Lazzer S, Pereira B, Fearnbach N, Montaurier C, Vermorel M, et al. Association of protein-energy partitioning with body weight and body composition changes in adolescents with severe obesity. Int J Obes 2005. 2022;46:2021–8.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3.
Lazzer S, Boirie Y, Poissonnier C, Petit I, Duché P, Taillardat M, et al. Longitudinal changes in activity patterns, physical capacities, energy expenditure, and body composition in severely obese adolescents during a multidisciplinary weight-reduction program. Int J Obes. 2005;29:37–46.
Lazzer S, Boirie Y, Montaurier C, Vernet J, Meyer M, Vermorel M. A weight reduction program preserves fat-free mass but not metabolic rate in obese adolescents. Obes Res. 2004;12:233–40.
Lazzer S, Vermorel M, Montaurier C, Meyer M, Boirie Y. Changes in adipocyte hormones and lipid oxidation associated with weight loss and regain in severely obese adolescents. Int J Obes 2005. 2005;29:1184–91.
Montaurier C, Richard R, Boirie Y. Two functional calorimetric chambers in france complete the Room Indirect Calorimetry Operating and Reporting Standards (RICORS) 1.0 Guide List. Obes Silver Spring Md. 2021;29:631.
Brouwer E. Report of the sub-committee on constants and factors. In: KL B (ed). New-York: Academic Press; 1965. p. 441–3.
Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301.
Favier J, Ripert J, Toque C, Feinberg M. Répertoire général des aliments. In: Table des composition. TEC&DOC Lavoisier. Paris: Lavoisier; 1996.
Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiol Camb Mass. 1992;3:143–55.
Cuenca-García M, Ortega FB, Ruiz JR, Labayen I, Moreno LA, Patterson E, et al. More physically active and leaner adolescents have higher energy intake. J Pediatr Janv. 2014;164:159–66.e2.
Fulton JE, Dai S, Steffen LM, Grunbaum JA, Shah SM, Labarthe DR. Physical activity, energy intake, sedentary behavior, and adiposity in youth. Am J Prev Med. 2009;37:S40–49.
Blundell JE, Finlayson G, Gibbons C, Caudwell P, Hopkins M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav. 2015;152:473–8.
Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance: effects of exercise. Am J Clin Nutr. 1987;45:296–306.
Flatt JP. The difference in the storage capacities for carbohydrate and for fat, and its implications in the regulation of body weight. Ann N Y Acad Sci. 1987;499:104–23.
Flatt JP. Glycogen levels and obesity. Int J Obes Relat Metab Disord. 1996;20:S1–11.
Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108:S105–112.
Mellinkoff SM, Frankland M, Boyle D, Greipel M. Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol. 1956;8:535–8.
Millward DJ. A protein-stat mechanism for regulation of growth and maintenance of the lean body mass. Nutr Res Rev. 1995;8:93–120.
Millward DJ. Metabolic demands for amino acids and the human dietary requirement: Millward and rRvers (1988) revisited. J Nutr. 1998;128:2563S–2576S.
Stubbs RJ. Peripheral signals affecting food intake. Nutr Burbank Los Angel Cty Calif. 1999;15:614–25.
Norris SA, Frongillo EA, Black MM, Dong Y, Fall C, Lampl M, et al. Nutrition in adolescent growth and development. Lancet Lond Engl 2022;399:172–84.
Boirie Y, Beaufrère B, Ritz P. Energetic cost of protein turnover in healthy elderly humans. Int J Obes Relat Metab Disord. 2001;25:601–5.
Simpson SJ, Raubenheimer D. The power of protein. Am J Clin Nutr. 2020;112:6–7.
Thivel D, Isacco L, Montaurier C, Boirie Y, Duché P, Morio B. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: a randomized controlled trial in calorimetric chambers. PLoS ONE. 2012;7:e29840.
Acknowledgements
The authors want to thank all the participants and the Romagnat Inpatient Pediatric Obesity Clinics.
Author information
Authors and Affiliations
Contributions
YB, SL, CM designed research and conducted research; DT, LI, BP, YD, HM analyzed data; and TD, LI, MH, JB, YB, SL, HM wrote the paper. TD, LI had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thivel, D., Hopkins, M., Lazzer, S. et al. Examining the roles of body composition, energy expenditure and substrate metabolism in the control of daily energy intake in adolescents with obesity. Int J Obes 49, 1076–1083 (2025). https://doi.org/10.1038/s41366-025-01740-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01740-6