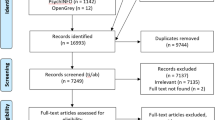
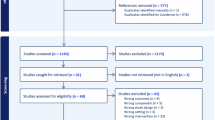
Abstract
Background/Objectives
One of the main challenges in weight loss is the dramatic interindividual variability in response to treatment. We aim to systematically identify factors relevant to weight loss effectiveness using machine learning (ML).
Subjects/Methods
We studied 1810 participants in the ONTIME program, which is based on cognitive-behavioral therapy for obesity (CBT-OB). We assessed 138 variables representing participants’ characteristics, clinical history, metabolic status, dietary intake, physical activity, sleep habits, chronotype, emotional eating, and social and environmental barriers to losing weight. We used XGBoost (extreme gradient boosting) to predict treatment response and SHAP (SHapley Additive exPlanations) to identify the most relevant factors for weight loss effectiveness.
Results
The total weight loss was 8.45% of the initial weight, the rate of weight loss was 543 g/wk., and attrition was 33%. Treatment duration (mean ± SD: 14.33 ± 8.61 weeks) and initial BMI (28.9 ± 3.33) were crucial factors for all three outcomes. The lack of motivation emerged as the most significant barrier to total weight loss and also influenced the rate of weight loss and attrition. Participants who maintained their motivation lost 1.4% more of their initial body weight than those who lost motivation during treatment (P < 0.0001). The second and third critical factors for decreased total weight loss were lower “self-monitoring” and “eating habits during treatment” (particularly higher snacking). Higher physical activity was a key variable for the greater rate of weight loss.
Conclusions
Machine learning analysis revealed key modifiable lifestyle factors during treatment, highlighting avenues for targeted interventions in future weight loss programs. Specifically, interventions should prioritize strategies to sustain motivation, address snacking behaviors, and enhance self-monitoring techniques. Further research is warranted to evaluate the efficacy of these strategies in improving weight loss outcomes.
Trial registration
clinicaltrials.gov: NCT02829619.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data sets generated during the current study are available from the corresponding authors upon reasonable request.
References
Yang HW, Garaulet M, Li P, Bandin C, Lin C, Lo MT, et al. Daily rhythm of fractal cardiac dynamics links to weight loss resistance: interaction with CLOCK 3111T/C genetic variant. Nutrients. 2021;13:2463.
Schutz Y, Montani JP, Dulloo AG. Low-carbohydrate ketogenic diets in body weight control: a recurrent plaguing issue of fad diets? Obes Rev. 2021;22:e13195.
Ostendorf DM, Blankenship JM, Grau L, Arbet J, Mitchell NS, Creasy SA, et al. Predictors of long-term weight loss trajectories during a behavioral weight loss intervention: An exploratory analysis. Obes Sci Pr. 2021;7:569–82.
Dashti HS, Scheer F, Saxena R, Garaulet M. Impact of polygenic score for BMI on weight loss effectiveness and genome-wide association analysis. Int J Obes (Lond). 2024;48:694–701.
Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–37.
Jacob A, Moullec G, Lavoie KL, Laurin C, Cowan T, Tisshaw C, et al. Impact of cognitive-behavioral interventions on weight loss and psychological outcomes: A meta-analysis. Health Psychol. 2018;37:417–32.
Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378:1485–92.
Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P, Behavioural Weight Management Review G. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557–68.
Dalle Grave R, Sartirana M, Calugi S. Personalized cognitive-behavioural therapy for obesity (CBT-OB): theory, strategies and procedures. Biopsychosoc Med. 2020;14:5.
Dalle Grave R, Melchionda N, Calugi S, Centis E, Tufano A, Fatati G, et al. Continuous care in the treatment of obesity: an observational multicentre study. J Intern Med. 2005;258:265–73.
Coughlin JW, Smith MT. Sleep, obesity, and weight loss in adults: Is there a rationale for providing sleep interventions in the treatment of obesity? Int Rev Psychiatry. 2014;26:177–88.
Vera B, Dashti HS, Gomez-Abellan P, Hernandez-Martinez AM, Esteban A, Scheer F, et al. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep. 2018;8:945.
Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond. 2013;37:604–11.
Jordan MI, Mitchell TM. Machine learning: Trends, perspectives, and prospects. Science. 2015;349:255–60.
A risk prediction model for type 2 diabetes based on weighted feature selection of random forest and xgboost ensemble classifier. 2019 eleventh international conference on advanced computational intelligence (ICACI). IEEE, 2019.
Application of XGBoost algorithm in hourly PM2. 5 concentration prediction. IOP conference series: earth and environmental science. IOP publishing, 2018.
Heymsfield SB, Thomas D, Nguyen AM, Peng JZ, Martin C, Shen W, et al. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev. 2011;12:e348–61.
Corbalan MD, Morales EM, Canteras M, Espallardo A, Hernandez T, Garaulet M. Effectiveness of cognitive-behavioral therapy based on the Mediterranean diet for the treatment of obesity. Nutrition. 2009;25:861–9.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023.
Garaulet M, Corbalan-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, et al. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110:917–21.
Perez-Llamas F, Garaulet M, Herrero F, Palma JT, Perez de Heredia F, Marin R, et al. [Multivalent informatics application for studies of the nutritional status of the population. Assessment of food intake]. Nutricion hospitalaria : organo oficial de la Soc Espanola de Nutricion Parenter y Enter. 2004;19:160–6.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Sanchez-Moreno C, Ordovas JM, Smith CE, Baraza JC, Lee YC, Garaulet M. APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J Nutr. 2011;141:380–5.
Shen J, Arnett DK, Peacock JM, Parnell LD, Kraja A, Hixson JE, et al. Interleukin1beta genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J Nutr. 2007;137:1846–51.
Lopez-Minguez J, Dashti HS, Madrid-Valero JJ, Madrid JA, Saxena R, Scheer F, et al. Heritability of the timing of food intake. Clin Nutr. 2019;38:767–73.
Knoops KT, Groot de LC, Fidanza F, Alberti-Fidanza A, Kromhout D, van Staveren WA. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: the HALE project. Eur J Clin Nutr. 2006;60:746–55.
Diller KR, Aggarwal SJ. Computer automated cell size and shape analysis in cryomicroscopy. J Microsc. 1987;146:209–19.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Garaulet M, Canteras M, Morales E, Lopez-Guimera G, Sanchez-Carracedo D, Corbalan-Tutau MD. Validation of a questionnaire on emotional eating for use in cases of obesity: the Emotional Eater Questionnaire (EEQ). Nutr Hosp. 2012;27:645–51.
Lundberg SM, Lee S-I A unified approach to interpreting model predictions. Advances in neural information processing systems 2017;30.
Pigsborg K, Kalea AZ, De Dominicis S, Magkos F. Behavioral and psychological factors affecting weight loss success. Curr Obes Rep. 2023;12:223–30.
Forrest LN, Ivezaj V, Grilo CM. Machine learning v. traditional regression models predicting treatment outcomes for binge-eating disorder from a randomized controlled trial. Psychol Med. 2023;53:2777–88.
Fisher A, Rudin C, Dominici F. All models are wrong, but many are useful: learning a variable’s importance by studying an entire class of prediction models simultaneously. J Mach Learn Res. 2019;20:1–81.
Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38.
Finkler E, Heymsfield SB, St-Onge MP. Rate of weight loss can be predicted by patient characteristics and intervention strategies. J Acad Nutr Diet. 2012;112:75–80.
Macaulay L, O’Dolan C, Avenell A, Carroll P, Cotton S, Dombrowski S, et al. Effectiveness and cost-effectiveness of text messages with or without endowment incentives for weight management in men with obesity (Game of Stones): study protocol for a randomised controlled trial. Trials. 2022;23:582.
Pirotta S, Joham A, Hochberg L, Moran L, Lim S, Hindle A, et al. Strategies to reduce attrition in weight loss interventions: A systematic review and meta-analysis. Obes Rev. 2019;20:1400–12.
Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I, Group D. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J Am Coll Nutr. 2009;28:159–68.
Burkhart PV, Sabate E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
Leyden E, Hanson P, Halder L, Rout L, Cherry I, Shuttlewood E, et al. Older age does not influence the success of weight loss through the implementation of lifestyle modification. Clin Endocrinol (Oxf). 2021;94:204–9.
Kim M, Yang J, Ahn WY, Choi HJ. Machine learning analysis to identify digital behavioral phenotypes for engagement and health outcome efficacy of an mhealth intervention for obesity: randomized controlled trial. J Med Internet Res. 2021;23:e27218.
Antoun J, Itani H, Alarab N, Elsehmawy A. The effectiveness of combining nonmobile interventions with the use of smartphone apps with various features for weight loss: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2022;10:e35479.
Mirkarimi K, Kabir MJ, Honarvar MR, Ozouni-Davaji RB, Eri M. Effect of motivational interviewing on weight efficacy lifestyle among women with overweight and obesity: a randomized controlled trial. Iran J Med Sci. 2017;42:187–93.
Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102.
Prestwich A, Kellar I, Parker R, MacRae S, Learmonth M, Sykes B, et al. How can self-efficacy be increased? Meta-analysis of dietary interventions. Health Psychol Rev. 2014;8:270–85.
Teodoro MC, Conceicao EM, de Lourdes M, Alves JR, Neufeld CB. Grazing’s frequency and associations with obesity, psychopathology, and loss of control eating in clinical and community contexts: A systematic review. Appetite. 2021;167:105620.
Zizza C, Siega-Riz AM, Popkin BM. Significant increase in young adults’ snacking between 1977-1978 and 1994-1996 represents a cause for concern! Prev Med. 2001;32:303–10.
Drummond S, Crombie N, Kirk T. A critique of the effects of snacking on body weight status. Eur J Clin Nutr. 1996;50:779–83.
Del Corral P, Chandler-Laney PC, Casazza K, Gower BA, Hunter GR. Effect of dietary adherence with or without exercise on weight loss: a mechanistic approach to a global problem. J Clin Endocrinol Metab. 2009;94:1602–7.
Acknowledgements
Grant PID2020-112768RB-I00 funded by MCIN/AEI/10.13039/501100011033. It was used to recruit participants, data collection, and analyses.
Author information
Authors and Affiliations
Contributions
Conceptualization: KH, MG; Formal analysis: H-W Y, HS and Y-Q Peng; Investigation: MG and KH; Data Curation: H-W Y; Writing - Original Draft: RDP-A, H-W Y, and MG; Visualization: RDP-A and H-WY; Writing - Review & Editing: MG, KH, FS, and HS; Supervision: M-T Lo, HS, MG, KH, FS.
Corresponding authors
Ethics declarations
Competing interests
FAJLS served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham. FAJLS interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare under their conflict of interest policies. FAJLS consultancies are not related to the current work. The other authors declare no conflicts of interest.
Ethics approval and consent to participate
Study procedures are described in clinicaltrials.gov: NCT02829619 and were approved by the Committee of Research Ethics of the University of Murcia (ID: 632/2017), and the protocol followed good clinical practice. Written informed consent for the publication of participants’ clinical details was obtained from the patient. A copy of the consent form is available for review by the Editor of this.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, HW., De la Peña-Armada, R., Sun, H. et al. Uncovering key factors in weight loss effectiveness through machine learning. Int J Obes 49, 1189–1199 (2025). https://doi.org/10.1038/s41366-025-01766-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01766-w