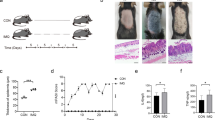
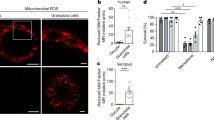
Abstract
Background/objective
Palmitic acid (PA) is known to be elevated in the follicular fluid of women with obesity, negatively affecting female fertility. However, the mechanism by which PA exposure reduces female fertility is not fully understood, and how it can be treated requires further investigation.
Methods
We first established in vivo and in vitro models of mouse oocyte maturation at high concentrations of PA and determined the effects of treatment with agomelatine (Ago) which is a melatonin receptor agonist with antioxidant properties. We assessed oocyte maturation rates, spindle morphology and chromosome morphology, oxidative stress and apoptosis levels. Lastly, we examined energy levels, mitochondrial function, and mitochondrial synthesis-related protein expression levels.
Results
Our results showed that PA exposure disrupted spindle assembly and chromosome alignment, reduced microtubule stability, and impaired the meiotic maturation of oocytes. PA also disrupted mitochondrial function, leading to decreased ATP production, elevated Reactive Oxygen Species(ROS) levels, oxidative stress, and apoptosis. Remarkably, Ago supplementation promoted oocyte quality by restoring spindle/chromosome conformation, maintaining mitochondrial function, lowering ROS levels, and inhibiting apoptosis.
Conclusions
This study establishes that Ago ameliorates metabolic stress-induced oocyte deterioration through mitochondrial functional restoration, providing mechanistic insights into obesity-associated infertility. Importantly, our study identifies a potentially favorable drug for combating obesity-induced female infertility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). Detailed individual analysis will be available from the corresponding author on reasonable request.
References
Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347–64. https://doi.org/10.1530/REP-09-0568.
Si C, Wang N, Wang M, Liu Y, Niu Z, Ding Z. TMT-based proteomic and bioinformatic analyses of human granulosa cells from obese and normal-weight female subjects. Reprod Biol Endocrinol. 2021;19:75. https://doi.org/10.1186/s12958-021-00760-x.
Niu ZH, Lin N, Gu RH, Sun YJ, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocr Metab. 2014;99:E2269–76. https://doi.org/10.1210/jc.2013-3942.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol. 2011;18:139–43. https://doi.org/10.1097/MED.0b013e3283444b09.
Palomer X, Pizarro-Delgado J, Barroso E, Vazquez-Carrera M. Palmitic and oleic acid: the Yin and Yang of Fatty acids in type 2 diabetes mellitus. Trends Endocrinol Metab. 2018;29:178–90. https://doi.org/10.1016/j.tem.2017.11.009.
Ding Y, Jiang YH, Zhu MJ, Zhu QL, He YQ, Lu Y, et al. Follicular fluid lipidomic profiling reveals potential biomarkers of polycystic ovary syndrome: a pilot study. Front Endocrinol. 2022;13. https://doi.org/10.3389/fendo.2022.960274.
Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–46. https://doi.org/10.1210/en.2010-0098.
Schatten H, Sun QY, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014;12:111. https://doi.org/10.1186/1477-7827-12-111.
Charalambous C, Webster A, Schuh M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat Rev Mol Cell Biol. 2023;24:27–44. https://doi.org/10.1038/s41580-022-00517-3.
van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P. Mitochondria: their relevance during oocyte ageing. Ageing Res Rev. 2021;70:101378. https://doi.org/10.1016/j.arr.2021.101378.
Marei WFA, De Bie J, Mohey-Elsaeed O, Wydooghe E, Bols PEJ, Leroy J. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol Reprod. 2017;96:1181–96. https://doi.org/10.1093/biolre/iox046.
Itami N, Shirasuna K, Kuwayama T, Iwata H. Palmitic acid induces ceramide accumulation, mitochondrial protein hyperacetylation, and mitochondrial dysfunction in porcine oocytes. Biol Reprod. 2018;98:644–53. https://doi.org/10.1093/biolre/ioy023.
Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. https://doi.org/10.14348/molcells.2016.2318.
Mohamed HE, Abo EDM, Mesbah NM, Saleh SM, Ali AA, Sakr AT. Raspberry ketone preserved cholinergic activity and antioxidant defense in obesity induced Alzheimer disease in rats. Biomed Pharmacother. 2018;107:1166–74. https://doi.org/10.1016/j.biopha.2018.08.034.
Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165838. https://doi.org/10.1016/j.bbadis.2020.165838.
Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19:486–94. https://doi.org/10.1093/molehr/gat026.
Naveed M, Li LD, Sheng G, Du ZW, Zhou YP, Nan S, et al. Agomelatine: an astounding sui-generis antidepressant?. Curr Mol Pharm. 2022;15:943–61. https://doi.org/10.2174/1874467214666211209142546.
Guardiola-Lemaitre B, De Bodinat C, Delagrange P, Millan MJ, Munoz C, Mocaer E. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharm. 2014;171:3604–19. https://doi.org/10.1111/bph.12720.
Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, Consolazione M, et al. Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int J Neuropsychopharmacol. 2013;16:323–38. https://doi.org/10.1017/S1461145711001970.
Moreno-SanJuan S, Puentes-Pardo JD, Casado J, Escudero-Feliu J, Khaldy H, Arnedo J, et al. Agomelatine, a melatonin-derived drug, as a new strategy for the treatment of colorectal cancer. Antioxidants. 2023;12. https://doi.org/10.3390/antiox12040926.
Maddukuri RK, Hema C, Sri Tejaswi K, Venkata Mounika M, Vegesana BP. Antidepressant efficacy of Agomelatine: meta-analysis of placebo controlled and active comparator studies. Asian J Psychiatr. 2021;65:102866. https://doi.org/10.1016/j.ajp.2021.102866.
Chumboatong W, Thummayot S, Govitrapong P, Tocharus C, Jittiwat J, Tocharus J. Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem Int. 2017;102:114–22. https://doi.org/10.1016/j.neuint.2016.12.011.
Aguiar CC, Almeida AB, Araujo PV, Vasconcelos GS, Chaves EM, do Vale OC, et al. Effects of agomelatine on oxidative stress in the brain of mice after chemically induced seizures. Cell Mol Neurobiol. 2013;33:825–35. https://doi.org/10.1007/s10571-013-9949-0.
Jia P, Liu C, Wu N, Jia D, Sun Y. Agomelatine protects against myocardial ischemia reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Transl Res. 2018;10:1310–23.
Feng X, Song Y, Sun Z, Loor JJ, Jiang Q, Gao C, et al. Palmitic acid hinders extracellular traps of neutrophil from postpartum dairy cow in vitro. J Dairy Sci. 2022;105:8286–97. https://doi.org/10.3168/jds.2021-21405.
Marei WFA, Van den Bosch L, Pintelon I, Mohey-Elsaeed O, Bols PEJ, Leroy J. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model. Hum Reprod. 2019;34:1984–98. https://doi.org/10.1093/humrep/dez161.
Wang Y, Pope I, Brennan-Craddock H, Poole E, Langbein W, Borri P, et al. A primary effect of palmitic acid on mouse oocytes is the disruption of the structure of the endoplasmic reticulum. Reproduction. 2021;163:45–56. https://doi.org/10.1530/REP-21-0332.
Jiang X, Pang Y, Zhao S, Hao H, Zhao X, Du W, et al. Thioredoxin-interacting protein regulates glucose metabolism and improves the intracellular redox state in bovine oocytes during in vitro maturation. Am J Physiol Endocrinol Metab. 2020;318:E405–16. https://doi.org/10.1152/ajpendo.00057.2019.
Guo Y, Sun J, Bu S, Li B, Zhang Q, Wang Q, et al. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell Cycle. 2020;19:1677–95. https://doi.org/10.1080/15384101.2020.1767403.
He X, Wang D, Zhu F, Jiang Y, Bi J, Lu X, et al. Astaxanthin alleviates palmitic acid-induced hindrance of porcine oocyte maturation. Reprod Domest Anim. 2022;57:1440–9. https://doi.org/10.1111/rda.14221.
Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, et al. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–95. https://doi.org/10.1530/rep.1.00735.
Wu T, Dong J, Fu J, Kuang Y, Chen B, Gu H, et al. The mechanism of acentrosomal spindle assembly in human oocytes. Science. 2022;378:eabq7361. https://doi.org/10.1126/science.abq7361.
Palazzo A, Ackerman B, Gundersen GG. Cell biology: tubulin acetylation and cell motility. Nature. 2003;421:230. https://doi.org/10.1038/421230a.
Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-α-induced alterations in metaphase II mouse oocyte spindle structure,. Fertil Steril. 2007;88:1220–31. https://doi.org/10.1016/j.fertnstert.2007.02.067.
Catandi GD, Cheng MH, Chicco AJ, Chen T, Carnevale EM. L-carnitine enhances developmental potential of bovine oocytes matured under high lipid concentrations in vitro. Anim Reprod Sci. 2023;252:107249. https://doi.org/10.1016/j.anireprosci.2023.107249.
Marei WFA, Van Raemdonck G, Baggerman G, Bols PEJ, Leroy J. Proteomic changes in oocytes after in vitro maturation in lipotoxic conditions are different from those in cumulus cells. Sci Rep. 2019;9:3673. https://doi.org/10.1038/s41598-019-40122-7.
Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res. 2018;65:e12514. https://doi.org/10.1111/jpi.12514.
Alruhaimi RS, Hassanein EHM, Bin-Jumah MN, Mahmoud AM. Cadmium cardiotoxicity is associated with oxidative stress and upregulated TLR-4/NF-kB pathway in rats; protective role of agomelatine. Food Chem Toxicol. 2023;180:114055. https://doi.org/10.1016/j.fct.2023.114055.
de Mello AH, Souza Lda R, Cereja AC, Schraiber Rde B, Florentino D, Martins MM, et al. Effect of subchronic administration of agomelatine on brain energy metabolism and oxidative stress parameters in rats. Psychiatry Clin Neurosci. 2016;70:159–66. https://doi.org/10.1111/pcn.12371.
Sun XC, Wang Y, Zeng HF, Xi YM, Lin H, Han ZY, et al. SIRT3 protects bovine mammary epithelial cells from heat stress damage by activating the AMPK signaling pathway. Cell Death Discov. 2021;7:304. https://doi.org/10.1038/s41420-021-00695-7.
Krishnaswamy R, Devaraj SN, Padma VV. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic Biol Med. 2010;49:50–60. https://doi.org/10.1016/j.freeradbiomed.2010.03.016.
Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod. 2016;31:2090–7. https://doi.org/10.1093/humrep/dew181.
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 2021;11:1845–63. https://doi.org/10.7150/thno.50905.
Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE. 2010;5:e10074. https://doi.org/10.1371/journal.pone.0010074.
Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–45. https://doi.org/10.1210/en.2010-0551.
Zhou YT, Li R, Li SH, Ma X, Liu L, Niu D, et al. Perfluorooctanoic acid (PFOA) exposure affects early embryonic development and offspring oocyte quality via inducing mitochondrial dysfunction. Environ Int. 2022;167:107413. https://doi.org/10.1016/j.envint.2022.107413.
Chang H, Li J, Zhang C, Qian W. Octocrylene exposure impairs mouse oocyte quality by inducing spindle defects and mitochondria dysfunction. Toxicology. 2022;479:153306. https://doi.org/10.1016/j.tox.2022.153306.
Harvey AJ. Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction. 2019;157:R159–79. https://doi.org/10.1530/REP-18-0431.
Chanmanee T, Wongpun J, Tocharus C, Govitrapong P, Tocharus J. The effects of agomelatine on endoplasmic reticulum stress related to mitochondrial dysfunction in hippocampus of aging rat model. Chem Biol Interact. 2022;351:109703. https://doi.org/10.1016/j.cbi.2021.109703.
Luptak M, Fisar Z, Hroudova J. Agomelatine, ketamine and vortioxetine attenuate energy cell metabolism-in vitro study. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms232213824.
Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29:2006–17. https://doi.org/10.1093/humrep/deu160.
Dong W, Yan L, Tan Y, Chen S, Zhang K, Gong Z, et al. Melatonin improves mitochondrial function by preventing mitochondrial fission in cadmium-induced rat proximal tubular cell injury via SIRT1-PGC-1alpha pathway activation. Ecotoxicol Environ Saf. 2022;242:113879. https://doi.org/10.1016/j.ecoenv.2022.113879.
Wang P, Zhang S, Lin S, Lv Z. Melatonin ameliorates diabetic hyperglycaemia-induced impairment of Leydig cell steroidogenic function through activation of SIRT1 pathway. Reprod Biol Endocrinol. 2022;20:117. https://doi.org/10.1186/s12958-022-00991-6.
Mahmoud AM, Abd El-Ghafar OAM, Alzoghaibi MA, Hassanein EHM. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARgamma and SIRT1. Life Sci. 2021;278:119600. https://doi.org/10.1016/j.lfs.2021.119600.
Acknowledgements
We thank Zhen Wang and Lei Chen (Life Science Research Core Services, Northwest A&F University, Yangling, China) for transmission electron microscopy experimental assistance. Mouse and syringe cartoon images used in figure were obtained from Scidraw.io.
Funding
This work was supported by the National Natural Science Foundation of China [grant number U24A20442]; the Key R&D and Transformation program of Qinghai Province [grant number 2023-NK-131, 2022-QY-209].
Author information
Authors and Affiliations
Contributions
RLZ and JMS conceptualized and designed the study. RLZ, YJT, YBZ and BZJ completed data collection. JWL, CSZ and WJS analysed and interpreted data. RLZ drafted the manuscript. JMS critically revised the manuscript for important intellectual content. CTZ and JMS supervised the study. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were conducted in strict accordance with the China National Guidelines for Ethical Review of Laboratory Animal Welfare (GB/T 35892-2018) and the Regulations for the Administration of Affairs Concerning Experimental Animals (2017 Revision). The experimental protocols were approved by the Animal Care and Use Committee of Northwest A&F University (Protocol number: XN-2023-1207). This study did not involve any human participants or human data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Tang, Y., Zhang, Y. et al. Agomelatine alleviates palmitic acid-induced mouse oocyte meiosis defects by restoring mitochondrial function. Int J Obes 49, 1781–1791 (2025). https://doi.org/10.1038/s41366-025-01825-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01825-2