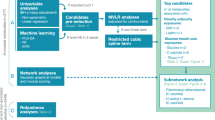
Abstract
Objectives
In this systematic review, we aimed to investigate the association of maternal body mass index (BMI) before or during pregnancy and the metabolic profiles of offspring in the life course.
Methods
We searched six databases: PubMed, Ovid Embase, MEDLINE, Web of Science, Wan Fang data and China National Knowledge Infrastructure, which yielded 3584 unduplicated articles, and after the full-text screening, 12 observational and three intervention studies met the inclusion criteria. Ten studies assessed pre-pregnancy BMI (ppBMI), whereas the remaining used booking BMI during antenatal visits. Most studies (10 out of 15) examined newborn metabolomics, four in early-mid childhood, and only one in adolescents and adults. The number of mother-offspring pairs ranged from 57 to 10,251, and the age of the population ranged from 15 to 44.
Results
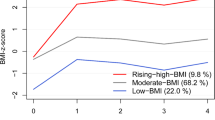
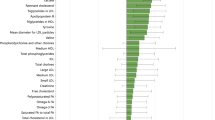
We found that maternal BMI before or during pregnancy was positively associated with adverse offspring metabolomic profiles, characterized by lipids (higher triglyceride, total cholesterol and lower high-density lipoprotein-cholesterol), amino acids (higher branched-chain amino acids and aromatic amino acids), carbohydrate-related metabolites (higher glucose and insulin). Studies on lipids have the most consistent results in both observational and intervention studies. The impact of maternal BMI on offspring metabolomics exerted from birth till adolescence and young adulthood.
Conclusions
Our study highlights a potential intergenerational association between maternal BMI and offspring metabolomic profiles. Interventions on maternal BMI before or during pregnancy showed the potential to reverse the adverse changes in offspring’s metabolic profiles, but long-term health benefits warrant to be verified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Denizli M, Capitano ML, Kua KL. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol. 2022;12:940937.
Isganaitis E, Venditti S, Matthews TJ, Lerin C, Demerath EW, Fields DA. Maternal obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am J Clin Nutr. 2019;110:111–20.
Patro Golab B, Santos S, Voerman E, Lawlor DA, Jaddoe VWV, Gaillard R, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health. 2018;2:812–21.
Reichetzeder C. Overweight and obesity in pregnancy: their impact on epigenetics. Eur J Clin Nutr. 2021;75:1710–22.
Hagström H, Simon TG, Roelstraete B, Stephansson O, Söderling J, Ludvigsson JF. Maternal obesity increases the risk and severity of NAFLD in offspring. J Hepatol. 2021;75:1042–8.
Iida M, Harada S, Takebayashi T. Application of metabolomics to epidemiological studies of atherosclerosis and cardiovascular disease. J Atheroscler Thromb. 2019;26:747–57.
McGarrah RW, Crown SB, Zhang G-F, Shah SH, Newgard CB. Cardiovascular metabolomics. Circ Res. 2018;122:1238–58.
Newgard CB. Metabolomics and metabolic diseases: where do we stand?. Cell Metab. 2017;25:43–56.
Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20:353–67.
Hu H, Jinrui Xiong JL, Yang Y, Chen Z, Liu M, Huang P. Maternal overweight/obesity and its impact on offspring metabolomic profiles. 2023. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023453599.
Kelly AC, Powell TL, Jansson T. Placental function in maternal obesity. Clin Sci. 2020;134:961–84.
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
StataCorp. Stata Statistical Software. College Station, TX: StataCorp LLC; 2023.
Zhang S. Study on the nutritional status of LCPUFA in pre-pregnancy overweight/obesity pregnant women and newborn infants. Anhui Medical University; Anhui: 2019.
Diana LSF, Dylan MW, Antti JK, Pasi S, Mika A-K, George Davey S, et al. Association of pre-pregnancy body mass index with offspring metabolic profile: analyses of 3 European prospective birth cohorts. PLoS Med. 2017;14:e1002376.
Tanvig M, Vinter CA, Jørgensen JS, Wehberg S, Ovesen PG, Beck-Nielsen H, et al. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years: results from the Lifestyle in Pregnancy and Offspring (LiPO) study. J Clin Endocrinol Metab. 2015;100:175–83.
Grotenfelt NE, Wasenius N, Eriksson JG, Huvinen E, Stach-Lempinen B, Koivusalo SB, et al. Effect of maternal lifestyle intervention on metabolic health and adiposity of offspring: findings from the Finnish Gestational Diabetes Prevention Study (RADIEL). Diab Metab. 2020;46:46–53.
Patel N, Hellmuth C, Uhl O, Godfrey K, Briley A, Welsh P, et al. Cord metabolic profiles in obese pregnant women: insights into offspring growth and body composition. J Clin Endocrinol Metab. 2018;103:346–55.
Shokry E, Marchioro L, Uhl O, Bermúdez MG, García-Santos JA, Segura MT, et al. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: results from the PREOBE cohort study. Acta Diabetol. 2019;56:421–30.
Yuan X, Ma Y, Wang J, Zhao Y, Zheng W, Yang R, et al. The influence of maternal prepregnancy weight and gestational weight gain on the umbilical cord blood metabolome: a case-control study. BMC Pregnancy Childbirth. 2024;24:297.
Lowe WL, Bain JR, Nodzenski M, Reisetter AC, Muehlbauer MJ, Stevens RD. et al. Maternal BMI and glycemia impact thefetal metabolome. Diab Care. 2017;40:902–10.
Duan Y. The impacts of maternal diabetes combined with obesity on offspring and their related molecular mechanisms. Tianjin Medical University; Tianjin: 2018.
Schlueter RJ, Al-Akwaa FM, Benny PA, Gurary A, Xie G, Jia W, et al. Prepregnant obesity of mothers in a multiethnic cohort is associated with cord blood metabolomic changes in offspring. J Proteome Res. 2020;19:1361–74.
Francis EC, Hunt KJ, Grobman WA, Skupski DW, Mani A, Hinkle SN. Maternal obesity and differences in child urine metabolome. Metabolites. 2024;14:574.
Uzun ÖÜ, Eneş D, Çınar M, Adugit AG, Uçar B, Duranoğlu A, et al. Cord blood metabolomic profiling in high risk newborns born to diabetic, obese, and overweight mothers: preliminary report. J Pediatr Endocrinol Metab. 2025;38:577–89.
Bucher M, Montaniel KRC, Myatt L, Weintraub S, Tavori H, Maloyan A. Dyslipidemia, insulin resistance, and impairment of placental metabolism in the offspring of obese mothers. J Dev Orig Health Dis. 2021;12:738–47.
Oostvogels AJJM, Stronks K, Roseboom TJ, van der Post JAM, van Eijsden M, Vrijkotte TGM. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5-6 years: the ABCD study. J Clin Endocrinol Metab. 2014;99:3845–54.
Perng W, Rifas-Shiman SL, McCulloch S, Chatzi L, Mantzoros C, Hivert M-F, et al. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism. 2017;76:11–22.
van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24.
Baas RE, Hutten BA, Henrichs J, Vrijkotte TGM. Associations between maternal lipid blood levels at 13 th week of pregnancy and offspring’s adiposity at age 11-12 years. J Clin Endocrinol Metab. 2022;107:dgac442.
Barbour LA, Hernandez TL. Maternal lipids and fetal overgrowth: making fat from fat. Clin Ther. 2018;40:1638–47.
Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13.
Kjeldsen EW, Nordestgaard LT, Frikke-Schmidt R. HDL cholesterol and non-cardiovascular disease: a narrative review. Int J Mol Sci. 2021;22:4547.
Dobiásová M, Frohlich J. Understanding the mechanism of LCAT reaction may help to explain the high predictive value of LDL/HDL cholesterol ratio. Physiol Res. 1998;47:387–97.
Stadler J, Poppel MV, Desoye G, Wadsack C, Marsche G. Obesity affects maternal and neonatal HDL metabolism and function. Pharmacology. 2023;379:S60.
Guijarro C, Cosín-Sales J. LDL cholesterol and atherosclerosis: the evidence. Clin Investig Arterioscler. 2021;33:25–32.
Avagliano L, Garò C, Marconi AM. Placental amino acids transport in intrauterine growth restriction. J Pregnancy. 2012;2012:972562.
Mansoori S, Ho MY, Ng KK, Cheng KK. Branched-chain amino acid metabolism: pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes Rev. 2025;26:e13856.
Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36.
Pana MP, Ayotte P, Anassour-Laouan-Sidi E, Suhas E, Gatti CMI, Lucas M. Branched-chain and aromatic amino acids in relation to fat mass and fat-free mass changes among adolescents: a school-based intervention. Metabolites. 2022;12:589.
Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1.
Moon JH, Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diab Metab J. 2022;46:3–14.
Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–68.
Gawlińska K, Gawliński D, Filip M, Przegaliński E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr Rev. 2021;79:709–2.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. https://doi.org/10.1371/journal.pmed1000097.
Funding
ML was funded by the Science and Technology Department of Jiangxi Province, the Natural Science Foundation of Jiangxi Province (20232BAB216103) and the Chunhui Program, Department of International Cooperation and Exchange, Ministry of Education (HZKY20220393), Nanchang University Youth Talent Cultivation Innovation Fund Project (XX202506050054) and the Chinese Scholarship Council.
Author information
Authors and Affiliations
Contributions
ML proposed the research idea, HH drafted the study proposal; JL, JX, ZC and HH conducted literature screening. YY and YZ drafted the tables, HH drafted the manuscript and RD ran the meta-analysis. ML, LL, YZ and PH reviewed and revised the manuscript. HH, YY and JL helped with reference collation. YZ helped with the paper submission. All authors approved the final publication of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, H., Yang, Y., Zhang, Y. et al. Intergenerational associations between maternal body mass index before or during pregnancy with offspring metabolomics: a systematic review and meta-analysis. Int J Obes (2025). https://doi.org/10.1038/s41366-025-01840-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-025-01840-3