Abstract
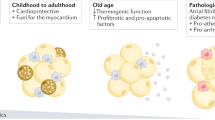
Epicardial adipose tissue (EAT), nestled directly against the beating heart, is a fascinating paradox in cardiovascular biology. In health, this unique fat depot functions as a metabolic ally and immune modulator, safeguarding the heart through energy support, anti-inflammatory actions, and mechanical cushioning. Yet, under pathological conditions, EAT transforms from a protective “heart-guardian” into a destructive “heart-harmer,” releasing pro-inflammatory mediators, driving oxidative stress, and fueling cardiovascular diseases like coronary artery disease (CAD) and atrial fibrillation (AF). This review explores EAT’s dual role, unraveling its complex biology and the delicate balance between protection and pathology. We delve into its cellular and molecular intricacies, highlight its pivotal contributions to cardiovascular health and disease, and synthesize cutting-edge research to illuminate its clinical relevance. By identifying current knowledge gaps and proposing future directions, we aim to inspire a deeper understanding of EAT and its potential as a therapeutic target. As we navigate this duality, EAT emerges as both a challenge and an opportunity in the quest to better understand and treat cardiovascular diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Le Jemtel TH, Samson R, Ayinapudi K, Singh T, Oparil S. Epicardial adipose tissue and cardiovascular disease. Curr Hypertens Rep. 2019;21:36.
Doukbi E, Soghomonian A, Sengenes C, Ahmed S, Ancel P, Dutour A, et al. Browning epicardial adipose tissue: friend or foe? Cells. 2022;11:991.
Lin A, Wong ND, Razipour A, McElhinney PA, Commandeur F, Cadet SJ, et al. Metabolic syndrome, fatty liver, and artificial intelligence-based epicardial adipose tissue measures predict long-term risk of cardiac events: a prospective study. Cardiovasc Diabetol. 2021;20:27.
Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017;22:889–902.
Couselo-Seijas M, Rodriguez-Manero M, Gonzalez-Juanatey JR, Eiras S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes Rev. 2021;22:e13277.
Abrishami A, Eslami V, Baharvand Z, Khalili N, Saghamanesh S, Zarei E, et al. Epicardial adipose tissue, inflammatory biomarkers and COVID-19: is there a possible relationship? Int Immunopharmacol. 2021;90:107174.
Ahmad I, Gupta S, Faulkner P, Mullens D, Thomas M, Sytha SP, et al. Single-nucleus transcriptomics of epicardial adipose tissue from female pigs reveals effects of exercise training on resident innate and adaptive immune cells. Cell Commun Signal. 2024;22:243.
Carena MC, Badi I, Polkinghorne M, Akoumianakis I, Psarros C, Wahome E, et al. Role of human epicardial adipose tissue-derived miR-92a-3p in myocardial redox state. J Am Coll Cardiol. 2023;82:317–32.
Santos D, Carvalho E. Adipose-related microRNAs as modulators of the cardiovascular system: the role of epicardial adipose tissue. J Physiol. 2022;600:1171–87.
Cho DH, Park SM. Epicardial adipose tissue and heart failure, friend or foe? Diabetes Metab J. 2024;48:373–84.
Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–61.
Yilmaz Z, Ince H, Aydin E, Yildirim Y, Yilmaz Aydin F, Yuksel E, et al. Relationship between epicardial adipose tissue and body composition as determined by multi-frequency bioelectrical impedance analysis in patients with stage 5 chronic kidney disease. Med Sci Monit. 2020;26:e920233.
Zhu J, Zhou W, Xie Z, Li W, Zhuo K. Impact of sex and menopausal status on the association between epicardial adipose tissue and diastolic function in patients with type 2 diabetes mellitus. Acad Radiol. 2023;30:823–32.
Shi KL, Qi L, Mao DB, Chen Y, Qian JY, Sun YB, et al. Impact of age on epicardial and pericoronary adipose tissue volume. Eur Rev Med. Pharmacol Sci. 2015;19:3257–65.
Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–9.
Peczkowski KK, Mashali MA, Saad NS, Hare A, Campbell CM, Whitson BA, et al. Quantification of Cardiac adipose tissue in failing and nonfailing human myocardium. J Am Heart Assoc. 2022;11:e025405.
Vural M, Dolek BA, Kilickap G, Kubra Bahadir G, Celal Gunes Y. Epicardial adipose tissue (EAT) thickness on non-gated chest CT as an alternative to EAT volume on cardiac CT. Acta Radiol. 2024;65:601–8.
Gac P, Hajdusianek W, Zorawik A, Macek P, Poreba M, Poreba R. Thickness and volume of epicardial adipose tissue in relation to stiffness and elasticity of aorta assessed by computed tomography angiography. Biomedicines. 2023;11:1617.
Uluca U, Demir F, Ece A, Sen V, Gunes A, Aktar F, et al. Assessment of epicardial adipose tissue thickness and the mean platelet volume in children with familial Mediterranean fever. Ital J Pediatr. 2015;41:15.
Malavazos AE, Iacobellis G, Dozio E, Basilico S, Di Vincenzo A, Dubini C, et al. Human epicardial adipose tissue expresses glucose-dependent insulinotropic polypeptide, glucagon, and glucagon-like peptide-1 receptors as potential targets of pleiotropic therapies. Eur J Prev Cardiol. 2023;30:680–93.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43.
Carbone AM, Del Calvo G, Nagliya D, Sharma K, Lymperopoulos A. Autonomic nervous system regulation of epicardial adipose tissue: potential roles for regulator of G protein signaling-4. Curr Issues Mol Biol. 2022;44:6093–103.
Rozsivalova K, Pierzynova A, Kratochvilova H, Lindner J, Lips M, Kotulak T, et al. Increased Number of Mast Cells in Epicardial Adipose Tissue of Cardiac Surgery Patients With Coronary Artery Disease. Physiol Res. 2020;69:621–31.
Vyas V, Sandhar B, Keane JM, Wood EG, Blythe H, Jones A, et al. Tissue-resident memory T cells in epicardial adipose tissue comprise transcriptionally distinct subsets that are modulated in atrial fibrillation. Nat Cardiovasc Res. 2024;3:1067–82.
Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58:248–55.
Caselli C, D’Amico A, Cabiati M, Prescimone T, Del Ry S, Giannessi D. Back to the heart: the protective role of adiponectin. Pharmacol Res. 2014;82:9–20.
Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18:631–44.
Samaha MM, El-Desoky MM, Hisham FA. AdipoRon, an adiponectin receptor agonist, modulates AMPK signaling pathway and alleviates ovalbumin-induced airway inflammation in a murine model of asthma. Int Immunopharmacol. 2024;136:112395.
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
Zhao Y, Shao C, Zhou H, Yu L, Bao Y, Mao Q, et al. Salvianolic acid B inhibits atherosclerosis and TNF-alpha-induced inflammation by regulating NF-kappaB/NLRP3 signaling pathway. Phytomedicine. 2023;119:155002.
Mahmood Z, Back M, Leanderson P, Thune R, Skoglund C, Jonasson L. Basal and exercise-induced expression of NLRP3 inflammasome-related components is increased in patients with chronic coronary syndrome. Atherosclerosis. 2025;405:119227.
Zang YH, Chen D, Zhou B, Chen AD, Wang JJ, Gao XY, et al. FNDC5 inhibits foam cell formation and monocyte adhesion in vascular smooth muscle cells via suppressing NFkappaB-mediated NLRP3 upregulation. Vascul Pharmacol. 2019;121:106579.
Zangi L, Oliveira MS, Ye LY, Ma Q, Sultana N, Hadas Y, et al. Insulin-like growth factor 1 receptor-dependent pathway drives epicardial adipose tissue formation after myocardial injury. Circulation. 2017;135:59–72.
Chen H, Liu L, Li M, Zhu D, Tian G. Epicardial adipose tissue-derived leptin promotes myocardial injury in metabolic syndrome rats through PKC/NADPH oxidase/ROS pathway. J Am Heart Assoc. 2023;12:e029415.
Fragasso G, Spoladore R, Bassanelli G, Cuko A, Montano C, Salerno A, et al. New directions in the treatment of heart failure: targeting free fatty acid oxidation. Curr Heart Fail Rep. 2007;4:236–42.
Han L, Liu J, Zhu L, Tan F, Qin Y, Huang H, et al. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 2018;17:185.
Nuutila P, Koivisto VA, Knuuti J, Ruotsalainen U, Teras M, Haaparanta M, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Investig. 1992;89:1767–74.
Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50.
Wahyuni T, Kobayashi A, Tanaka S, Miyake Y, Yamamoto A, Bahtiar A, et al. Maresin-1 induces cardiomyocyte hypertrophy through IGF-1 paracrine pathway. Am J Physiol Cell Physiol. 2021;321:C82–C93.
Samarel AM. IGF-1 overexpression rescues the failing heart. Circ Res. 2002;90:631–3.
Zaman R, Hamidzada H, Kantores C, Wong A, Dick SA, Wang Y, et al. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress. Immunity. 2021;54:2057–71.e6.
Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013–22.
Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab. 2013;24:408–20.
D’Marco L, Puchades MJ, Gorriz JL, Romero-Parra M, Lima-Martinez M, Soto C, et al. Epicardial adipose tissue, adiponectin and leptin: a potential source of cardiovascular risk in chronic kidney disease. Int J Mol Sci. 2020;21:978.
Palanivel R, Ganguly R, Turdi S, Xu A, Sweeney G. Adiponectin stimulates Rho-mediated actin cytoskeleton remodeling and glucose uptake via APPL1 in primary cardiomyocytes. Metabolism. 2014;63:1363–73.
Gao X, Jakovljevic DG, Beard DA. Cardiac metabolic limitations contribute to diminished performance of the heart in aging. Biophys J. 2019;117:2295–302.
Luo P, Zheng M, Zhang R, Zhang H, Liu Y, Li W, et al. S-Allylmercaptocysteine improves alcoholic liver disease partly through a direct modulation of insulin receptor signaling. Acta Pharm Sin B. 2021;11:668–79.
Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71.
Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J. Aging and autophagy in the Heart. Circ Res. 2016;118:1563–76.
Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care. 2011;34:730–3.
Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-gamma/adiponectin signalling. Circ Res. 2016;118:842–55.
Klein M, Varga I. Microenvironment of immune cells within the visceral adipose tissue sensu lato vs. epicardial adipose tissue: what do we know? Inflammation. 2018;41:1142–56.
He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21.
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776.
Furuhashi M, Fuseya T, Murata M, Hoshina K, Ishimura S, Mita T, et al. Local production of fatty acid-binding protein 4 in epicardial/perivascular fat and macrophages is linked to coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:825–34.
Choy M, Huang Y, Peng Y, Liang W, He X, Chen C, et al. Association between epicardial adipose tissue and incident heart failure mediating by alteration of natriuretic peptide and myocardial strain. BMC Med. 2023;21:117.
Li T, Li X, Feng Y, Dong G, Wang Y, Yang J. The role of matrix metalloproteinase-9 in atherosclerotic plaque instability. Mediat Inflamm. 2020;2020:3872367.
Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–69.
Santoro A, Kahn BB. Adipocyte regulation of insulin sensitivity and the risk of type 2 diabetes. N Engl J Med. 2023;388:2071–85.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28.
Naryzhnaya NV, Koshelskaya OA, Kologrivova IV, Kharitonova OA, Evtushenko VV, Boshchenko AA. Hypertrophy and insulin resistance of epicardial adipose tissue adipocytes: association with the coronary artery disease severity. Biomedicines. 2021;9:64.
Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556:505–9.
Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51.
Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–75.
Edsen F, Habib P, Matz O, Nikoubashman O, Wiesmann M, Frick M, et al. Epicardial adipose tissue thickness assessed by CT is a marker of atrial fibrillation in stroke patients. Ann Clin Transl Neurol. 2022;9:1668–72.
Zhu R, Wang W, Gao Y, Wang J, Li B, Cheng Z, et al. Insulin resistance aggravates myocardial fibrosis in non-diabetic hypertensive patients by altering the function of epicardial adipose tissue: a cardiac magnetic resonance study. Diabetol Metab Syndr. 2025;17:133.
Krishnan A, Chilton E, Raman J, Saxena P, McFarlane C, Trollope AF, et al. Are interactions between epicardial adipose tissue, cardiac fibroblasts and cardiac myocytes instrumental in atrial fibrosis and atrial fibrillation? Cells. 2021;10:2501.
Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. 2017;3:425–35.
Yang X, An N, Zhong C, Guan M, Jiang Y, Li X, et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30:101432.
Hao S, Sui X, Wang J, Zhang J, Pei Y, Guo L, et al. Secretory products from epicardial adipose tissue induce adverse myocardial remodeling after myocardial infarction by promoting reactive oxygen species accumulation. Cell Death Dis. 2021;12:848.
Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76:1197–211.
Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021;143:2475–93.
van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail. 2022;24:2238–50.
Ran CQ, Su Y, Li J, Wu K, Liu ZL, Yang Y, et al. Epicardial adipose tissue volume highly correlates with left ventricular diastolic dysfunction in endogenous Cushing’s syndrome. Ann Med. 2024;56:2387302.
Abdin A, Bohm M, Shahim B, Karlstrom P, Kulenthiran S, Skouri H, et al. Heart failure with preserved ejection fraction epidemiology, pathophysiology, diagnosis and treatment strategies. Int J Cardiol. 2024;412:132304.
Anthony SR, Guarnieri AR, Gozdiff A, Helsley RN, Phillip Owens A, Tranter M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin Sci. 2019;133:2329–44.
Chen D, Zhang Y, Yidilisi A, Xu Y, Dong Q, Jiang J. Causal associations between circulating adipokines and cardiovascular disease: a Mendelian randomization study. J Clin Endocrinol Metab. 2022;107:e2572–80.
Liao GZ, Liu HH, He CH, Feng JY, Zhuang XF, Wang JX, et al. Free fatty acids: independent predictors of long-term adverse cardiovascular outcomes in heart failure patients. Lipids Health Dis. 2024;23:343.
Packer M. Drugs That Ameliorate Epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J Card Fail. 2019;25:986–1003.
Jian M, Kwan JS, Bunting M, Ng RC, Chan KH. Adiponectin suppresses amyloid-beta oligomer (AbetaO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-kappaB signaling pathway. J Neuroinflammation. 2019;16:110.
Wang L, Luo Y, Luo L, Wu D, Ding X, Zheng H, et al. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibition of IL-33 signaling. J Exp Med. 2021;218:e20191054.
Ma YL, Xu M, Cen XF, Qiu HL, Guo YY, Tang QZ. Tectorigenin protects against cardiac fibrosis in diabetic mice heart via activating the adiponectin receptor 1-mediated AMPK pathway. Biomed Pharmacother. 2024;174:116589.
Thankam FG, Agrawal DK. Single cell genomics identifies unique cardioprotective phenotype of stem cells derived from epicardial adipose tissue under ischemia. Stem Cell Rev Rep. 2022;18:294–335.
Bianchi VE. Impact of nutrition on cardiovascular function. Curr Probl Cardiol. 2020;45:100391.
Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–66.
Horckmans M, Bianchini M, Santovito D, Megens RTA, Springael JY, Negri I, et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation. 2018;137:948–60.
Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, et al. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39:724–38.
Tu Q, Liu S, Chen T, Li Z, Lin D. Effects of adiponectin on random pattern skin flap survival in rats. Int Immunopharmacol. 2019;76:105875.
Konwerski M, Gasecka A, Opolski G, Grabowski M, Mazurek T. Role of epicardial adipose tissue in cardiovascular diseases: a review. Biology. 2022;11:355.
Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–72.
Liu Z, Wang S, Wang Y, Zhou N, Shu J, Stamm C, et al. Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis. 2019;284:230–6.
Filtz A, Lorenzatti D, Scotti A, Pina P, Fernandez-Hazim C, Huang D, et al. Relationship between epicardial adipose tissue and coronary atherosclerosis by CCTA in young adults (18-45). Am J Prev Cardiol. 2024;19:100711.
Madonna R, Massaro M, Scoditti E, Pescetelli I, De Caterina R. The epicardial adipose tissue and the coronary arteries: dangerous liaisons. Cardiovasc Res. 2019;115:1013–25.
Iacobellis G. Aging effects on epicardial adipose tissue. Front Aging. 2021;2:666260.
White IA. Cardiac Sympathetic denervation in the failing heart: a role for epicardial adipose tissue. Circ Res. 2016;118:1189–91.
Benedetti F, Davinelli S, Krishnan S, Gallo RC, Scapagnini G, Zella D, et al. Sulfur compounds block MCP-1 production by Mycoplasma fermentans-infected macrophages through NF-kappaB inhibition. J Transl Med. 2014;12:145.
Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, et al. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2.
Weber C, Habenicht AJR, von Hundelshausen P. Novel mechanisms and therapeutic targets in atherosclerosis: inflammation and beyond. Eur Heart J. 2023;44:2672–81.
Yan LS, Zhang SF, Luo G, Cheng BC, Zhang C, Wang YW, et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism. 2022;131:155200.
Li Y, Munoz-Mayorga D, Nie Y, Kang N, Tao Y, Lagerwall J, et al. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK. Cell Metab. 2024;36:1351–70.e8.
Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci ((Lond)). 2013;124:491–507.
Wang L, Ye X, Hua Y, Song Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed Pharmacother. 2018;105:121–9.
Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–70.
Decleves AE, Zolkipli Z, Satriano J, Wang L, Nakayama T, Rogac M, et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 2014;85:611–23.
Janani C, Ranjitha Kumari BD. PPAR gamma gene-a review. Diabetes Metab Syndr. 2015;9:46–50.
Martinez Calejman C, Trefely S, Entwisle SW, Luciano A, Jung SM, Hsiao W, et al. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Nat Commun. 2020;11:575.
Ju Z, Su M, Hong J, Kim E, Jung JH. Anti-inflammatory effects of an optimized PPAR-gamma agonist via NF-kappaB pathway inhibition. Bioorg Chem. 2020;96:103611.
Liu C, Xu X, He X, Ren J, Chi M, Deng G, et al. Activation of the Nrf-2/HO-1 signalling axis can alleviate metabolic syndrome in cardiovascular disease. Ann Med. 2023;55:2284890.
Huang CY, Deng JS, Huang WC, Jiang WP, Huang GJ. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 2020;12:1742.
Han J, Shi X, Xu J, Lin W, Chen Y, Han B, et al. DL-3-n-butylphthalide prevents oxidative stress and atherosclerosis by targeting Keap-1 and inhibiting Keap-1/Nrf-2 interaction. Eur J Pharm Sci. 2022;172:106164.
El-Sahar AE, Bekhit N, Eissa NM, Abdelsalam RM, Essam RM. Targeting HMGB1/PI3K/Akt and NF-kappaB/Nrf-2 signaling pathways by vildagliptin mitigates testosterone-induced benign prostate hyperplasia in rats. Life Sci. 2023;322:121645.
Calcaterra V, Cena H, Garella V, Loperfido F, Chillemi C, Manuelli M, et al. Assessment of epicardial fat in children: its role as a cardiovascular risk factor and how it is influenced by lifestyle habits. Nutrients. 2024;16:420.
van Eyk HJ, van Schinkel LD, Kantae V, Dronkers CEA, Westenberg JJM, de Roos A, et al. Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr Diabetes. 2018;8:6.
Barrio-Lopez MT, Ruiz-Canela M, Goni L, Valiente AM, Garcia SR, de la OV, et al. Mediterranean diet and epicardial adipose tissue in patients with atrial fibrillation treated with ablation: a substudy of the ‘PREDIMAR’ trial. Eur J Prev Cardiol. 2024;31:348–55.
Christensen RH, Wedell-Neergaard AS, Lehrskov LL, Legaard GE, Dorph E, Larsen MK, et al. Effect of aerobic and resistance exercise on cardiac adipose tissues: secondary analyses from a randomized clinical trial. JAMA Cardiol. 2019;4:778–87.
Launbo N, Zobel EH, von Scholten BJ, Faerch K, Jorgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: a systematic review and meta-analysis. Obes Rev. 2021;22:e13136.
Angulo J, El Assar M, Alvarez-Bustos A, Rodriguez-Manas L. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35:101513.
Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19:593–606.
Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022;21:169.
She J, Tuerhongjiang G, Guo M, Liu J, Hao X, Guo L, et al. Statins aggravate insulin resistance through reduced blood glucagon-like peptide-1 levels in a microbiota-dependent manner. Cell Metab. 2024;36:408–21.e5.
Bray JJH, Foster-Davies H, Salem A, Hoole AL, Obaid DR, Halcox JPJ, et al. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes Obes Metab. 2021;23:1806–22.
Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81.
Sheikhbahaei E, Tavassoli Naini P, Agharazi M, Pouramini A, Rostami S, Bakhshaei S, et al. Cardiac fat pat change after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. Surg Obes Relat Dis. 2023;19:653–64.
Sandoval DA, Patti ME. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol. 2023;19:164–76.
Iannelli A, Anty R, Schneck AS, Tran A, Hebuterne X, Gugenheim J. Evolution of low-grade systemic inflammation, insulin resistance, anthropometrics, resting energy expenditure and metabolic syndrome after bariatric surgery: a comparative study between gastric bypass and sleeve gastrectomy. J Visc Surg. 2013;150:269–75.
Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes. 2016;40:275–80.
Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–50.
Gashi G, Madoerin P, Maushart CI, Michel R, Senn JR, Bieri O, et al. MRI characteristics of supraclavicular brown adipose tissue in relation to cold-induced thermogenesis in healthy human adults. J Magn Reson Imaging. 2019;50:1160–8.
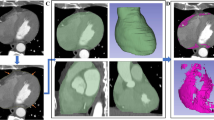
West HW, Siddique M, Williams MC, Volpe L, Desai R, Lyasheva M, et al. Deep-learning for epicardial adipose tissue assessment with computed tomography: implications for cardiovascular risk prediction. JACC Cardiovasc Imaging. 2023;16:800–16.
Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–25.
Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110:534–8.
Fukushima T, Maetani T, Chubachi S, Tanabe N, Asakura T, Namkoong H, et al. Epicardial adipose tissue measured from analysis of adipose tissue area using chest CT imaging is the best potential predictor of COVID-19 severity. Metabolism. 2024;150:155715.
Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–4.
Hassan M, Said K, Rizk H, ElMogy F, Donya M, Houseni M, et al. Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging. 2016;17:1169–77.
Sang C, Hu X, Zhang D, Shao Y, Qiu B, Li C, et al. The predictive value of left atrium epicardial adipose tissue on recurrence after catheter ablation in patients with different types of atrial fibrillation. Int J Cardiol. 2023;379:33–39.
Wang X, Leng S, Adamson PD, Greer CE, Huang W, Lee HK, et al. Characterizing cardiac adipose tissue in post-acute myocardial infarction patients via CT imaging: a comparative cross-sectional study. Eur Heart J Cardiovasc Imaging. 2025;26:733–40.
Hammache N, Pegorer-Sfes H, Benali K, Magnin Poull I, Olivier A, Echivard M, et al. Is There an Association between Epicardial Adipose Tissue and Outcomes after Paroxysmal Atrial Fibrillation Catheter Ablation?. J. Clin. Med. 2021;10:3037.
Acknowledgements
We are grateful to all of our colleagues from our research group who provided insightful feedback and suggestions during the preparation of this review.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 32400767) and General Program of Basic Science (Natural Sciences) Research for Higher Education Institutions in Jiangsu Province (24KJB180001).
Author information
Authors and Affiliations
Contributions
Yu Tian: Writing—original draft, Visualization. Pingping Wang: Supervision, Writing—Review and editing. Zhifeng Dong: Project administration, Conceptualization. Yu Tian and Pingping Wang contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, Y., Wang, P. & Dong, Z. Heart-guarding or heart-harming? The dual role of epicardial adipose tissue in cardiovascular health and disease. Int J Obes 49, 2156–2167 (2025). https://doi.org/10.1038/s41366-025-01852-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01852-z