Abstract
Background/objectives
This study investigated the efficacy of a novel multi-strain probiotic (MSP), composed of Limosilactobacillus fermentum BAB 7912, Bacillus rugosus PIC5CR, and Bacillus rugosus PIB9CR, in preventing and reverting diet-induced obesity in Balb/c male mice.
Subjects/methods
This study used 8-week-old Balb/c mice. A total of 40 mice were divided into five groups namely control negative (CN), control with obesity (CO), and three treatment groups: microbial consortium treated (MCT), Healthy control 1 (HC1), and Healthy control 2 (HC2). Obesity was induced using a high-fat diet. MSP formulation developed indigenously as part of previous study, was fed to Balb/c mice at different time intervals to study its preventive and ameliorative potential. Animals were dissected for the collection of blood as well as various organs to study the effect of MSP feeding on obesity status. Results were validated using histopathological and metagenomic data.
Results
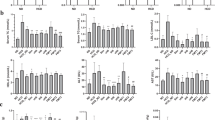
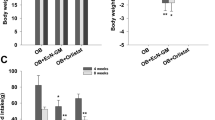
The CN and other treatment groups gained significant weight at the end of 6 weeks, while no significant weight gain was observed among HC1 group animals that were fed with HFD and MSP together. This highlights the preventive effect of continuous MSP feeding in the HC1 animal group. Initial liver histopathology in the HC1 group revealed enlarged hepatocytes and fat droplets. By week 9, the MCT group, which received MSP with a basal diet, showed liver recovery towards normal, accompanied by body weight improvement from 28.02 ± 0.7 g to 26.18 ± 0.96 g. Metagenomic analysis revealed that MSP treatment increased the relative abundance of health-promoting bacteria, notably Lactobacillaceae (specifically Lactobacillus).
Conclusions
Findings indicated that continuous consumption of MSP contributes significantly in prevention of obesity and associated metabolic disorders. Future studies are needed to explore the mechanisms underlying these effects and to evaluate the potential of MSP for human health.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions, although supplementary data have been provided for the deep understanding of findings to the readers.
References
Bandyopadhyay B, Das S, Mitra PK, Kundu A, Mandal V, Adhikary R, et al. Characterization of two new strains of Lactococcus lactis for their probiotic efficacy over commercial synbiotics consortia. Braz J Microbiol. 2022;53:903–20. https://doi.org/10.1007/s42770-022-00685-6.
Rautmann AW, de La Serre CB. Microbiota’s role in diet-driven alterations in food intake: satiety, energy balance, and reward. Nutrients. 2021;13:3067 https://doi.org/10.3390/nu13093067.
Mori P, Chauhan M, Modasiya I, Kumar V. Dietary modulation of the nervous and immune system: role of Probiotics/Prebiotics/Synbiotics/Postbiotics. In: Kothari, V., Kumar, P., Ray, S, editors. Probiotics, prebiotics, synbiotics, and postbiotics. Singapore: Springer; 2023:307–28. https://doi.org/10.1007/978-81-99-1463-0_16.
Okochi M, Sugita T, Asai Y, Tanaka M, Honda H. Screening of peptides associated with adhesion and aggregation of Lactobacillus rhamnosus GG in vitro. Biochem Eng J. 2017;128:178–85. https://doi.org/10.1016/j.bej.2017.10.004.
Falah F, Vasiee A, Behbahani BA, Yazdi FT, Moradi S, Mortazavi SA, et al. Evaluation of adherence and anti-infective properties of probiotic Lactobacillus fermentum strain 4–17 against Escherichia coli causing urinary tract infection in humans. Micro Pathog. 2019;131:246–53. https://doi.org/10.1016/j.micpath.2019.04.006.
Andriantsoanirina V, Teolis AC, Xin LX, Butel MJ, Aires J. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: comparison of cell surface properties. Anaerobe. 2014;28:212–5. https://doi.org/10.1016/j.anaerobe.2014.07.002.
Zuo F, Yu R, Feng X, Chen L, Zeng Z, Khaskheli GB, et al. Characterization and in vitro properties of potential probiotic Bifidobacterium strains isolated from breast-fed infant feces. Ann Microbiol. 2016;66:1027–37. https://doi.org/10.1007/s13213-015-1187-x.
Krausova G, Hyrslova I, Hynstova I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation. 2019;5:100. https://doi.org/10.3390/fermentation5040100.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. https://doi.org/10.1038/nature05485.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. https://doi.org/10.1016/s0140-6736(05)66378-7.
Chauhan M, Mori P, Kumar V. Human microbiome in malnutrition. In: Kothari, V., Kumar, P., Ray, S. editors. Probiotics, prebiotics, synbiotics, and postbiotics. Singapore: Springer; 2023:81–100. https://doi.org/10.1007/978-81-99-1463-0_5.
Stoner L, Cornwall J. Did the American Medical Association make the correct decision classifying obesity as a disease? Australas Med J. 2014;7:462 https://doi.org/10.4066/amj.2014.2281.
Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–84. https://doi.org/10.2337/dc10-0556.
Russo M, Marquez A, Abeijón-Mukdsi MC, Santacruz A, López-Malo A, Gauffin-Cano P, et al. Microencapsulated feruloyl esterase-producing lactobacilli ameliorate lipid profile and glycaemia in high-fat diet-induced obese mice. Benef Microbes. 2019;10:189–98. https://doi.org/10.3920/bm2018.0025.
Maniya H, Chauhan M, Kumar V. Organic acids of microbial origin as nutraceuticals. In: Kothari, V., Ray, S., Kumar, P, editors. Microbial products for health and nutrition. Singapore: Springer; 2024:109–30. https://doi.org/10.1007/978-81-97-4235-6_5.
Baboota RK, Bishnoi M, Ambalam P, Kondepudi KK, Sarma SM, Boparai RK, et al. Functional food ingredients for the management of obesity and associated co-morbidities–a review. J Funct Foods. 2013;5:997–1012. https://doi.org/10.1016/j.jff.2013.04.014.
Xie L, Su H, Sun C, Zheng X, Chen W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci Technol. 2018;72:13–24. https://doi.org/10.1016/j.tifs.2017.12.002.
Roselli M, Finamore A, Brasili E, Rami R, Nobili F, Orsi C, et al. Beneficial effects of a selected probiotic mixture administered to high-fat-fed mice before and after the development of obesity. J Funct Foods. 2018;45:321–9. https://doi.org/10.1016/j.jff.2018.03.039.
Lin HL, Shiu YL, Chiu CS, Huang SL, Liu CH. Screening probiotic candidates for a mixture of probiotics to enhance the growth performance, immunity, and disease resistance of Asian seabass, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish Shellfish Immunol. 2017;60:474–82. https://doi.org/10.1016/j.fsi.2016.11.026.
Ouwehand AC, Invernici MM, Furlaneto FA, Messora MR. Effectiveness of multi-strain versus single-strain probiotics: current status and recommendations for the future. J Clin Gastroenterol. 2018;52:S35–S40. https://doi.org/10.1097/mcg.0000000000001052.
Kwoji ID, Aiyegoro OA, Okpeku M, Adeleke MA. Multi-strain probiotics: synergy among isolates enhances biological activities. Biology. 2021;10:322. https://doi.org/10.3390/biology10040322.
Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219–33. https://doi.org/10.1016/j.ijfoodmicro.2004.05.012.
Puvanasundram P, Chong CM, Sabri S, Yusoff MSM, Lim KC, Karim M. Efficacy of single and multi-strain probiotics on in vitro strain compatibility, pathogen inhibition, biofilm formation capability, and stress tolerance. Biology. 2022;11:1644. https://doi.org/10.3390/biology11111644.
Maniya H, Modasiya I, Chauhan M, Mori P, Kumar V. Developing robust probiotic consortia: a methodological optimization approach. Curr Microbiol. 2024;81:1–12. https://doi.org/10.1007/s00284-024-03933-0.
Modasiya I, Mori P, Maniya H, Chauhan M, Grover C, Kumar V, et al. In vitro screening of bacterial isolates from dairy products for probiotic properties and other health-promoting attributes. Food Sci Nutr. 2024;12:10756–69. https://doi.org/10.1002/fsn3.4537.
Chauhan M, Modasiya I, Maniya H, Mori P, Kumar V. Assessment of multi-strain probiotic exhibiting in vitro cholesterol-lowering, antioxidative and lipolytic properties. Microbe. 2025;6:100280. https://doi.org/10.1016/j.microb.2025.100280.
Fabersani E, Russo M, Marquez A, Abeijón-Mukdsi C, Medina R, Gauffin-Cano P. Modulation of intestinal microbiota and immunometabolic parameters by caloric restriction and lactic acid bacteria. Food Res Int. 2019;124:188–99. https://doi.org/10.1016/j.foodres.2018.06.014.
Michael DR, Davies TS, Moss JWE, Calvente DL, Ramji DP, Marchesi JR, et al. The anti-cholesterolaemic effect of a consortium of probiotics: an acute study in C57BL/6J mice. Sci Rep. 2017;7:2883. https://doi.org/10.1038/s41598-017-02889-5.
Gan Y, Tang MW, Tan F, Zhou XR, Fan L, Xie YX, et al. Anti-obesity effect of Lactobacillus plantarum CQPC01 by modulating lipid metabolism in high-fat diet-induced C57BL/6 mice. J Food Biochem. 2020;44:e13491. https://doi.org/10.1111/jfbc.13491.
Darwish AM, Mabrouk DM, Desouky HM, Khattab AEN. Evaluation of the effectiveness of two new strains of Lactobacillus on obesity-induced kidney diseases in BALB/c mice. J Genet Eng Biotechnol. 2022;20:148. https://doi.org/10.1186/s43141-022-00427-z.
Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2018;60:175–84. https://doi.org/10.1016/j.nut.2018.10.002.
Filannino P, Di Cagno R, Gobbetti M. Metabolic and functional paths of lactic acid bacteria in plant foods: get out of the labyrinth. Curr Opin Biotechnol. 2018;49:64–72. https://doi.org/10.1016/j.copbio.2017.07.016.
Venegas-Ortega MG, Flores-Gallegos AC, Martínez-Hernández JL, Aguilar CN, Nevárez-Moorillón GV. Production of bioactive peptides from lactic acid bacteria: a sustainable approach for healthier foods. Compr Rev Food Sci Food Saf. 2019;18:1039–51. https://doi.org/10.1111/1541-4337.12455.
Das TK, Kar P, Panchali T, Khatun A, Dutta A, Ghosh S, et al. Anti-obesity potentiality of Lactiplantibacillus plantarum E2_MCCKT isolated from a fermented beverage, haria: a high fat diet-induced obese mice model study. World J Microbiol Biotechnol. 2024;40:168 https://doi.org/10.1007/s11274-024-03983-3.
Zhu K, Tan F, Mu J, Yi R, Zhou X, Zhao X. Anti-obesity effects of Lactobacillus fermentum CQPC05 isolated from Sichuan pickle in high-fat diet-induced obese mice through PPAR-α signaling pathway. Microorganisms. 2019;7:194 https://doi.org/10.3390/microorganisms7070194.
Fabersani E, Marquez A, Russo M, Ross R, Torres S, Fontana C, et al. Lactic acid bacteria strains differently modulate gut microbiota and metabolic and immunological parameters in high-fat diet-fed mice. Front Nutr. 2021;8:718564. https://doi.org/10.3389/fnut.2021.718564.
Li J, Wu H, Liu Y, Yang L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp Anim. 2020;69:326–35. https://doi.org/10.1538/expanim.19-0148.
Wu CS, Lin CC, Hsieh FC, Wu TY, Fang AH. Antiobesity effect of Lacticaseibacillus paracasei LM-141 on high-fat diet-induced rats through alleviation of inflammation and insulin resistance. J Evid Based Complement Altern Med. 2023;2023:1011591. https://doi.org/10.1155/2023/1011591.
Parto P, Lavie CJ. Obesity and cardiovascular diseases. Curr Probl Cardiol. 2017;42:376–94. https://doi.org/10.1016/j.cpcardiol.2017.04.004.
Liang X, Lv Y, Zhang Z, Yi H, Liu T, Li R, et al. Study on intestinal survival and cholesterol metabolism of probiotics. LWT. 2020;124:109132. https://doi.org/10.1016/j.lwt.2020.109132.
Al Zarzour RH, Ahmad M, Asmawi MZ, Kaur G, Saeed MAA, Al-Mansoub MA, et al. Phyllanthus niruri standardized extract alleviates the progression of non-alcoholic fatty liver disease and decreases atherosclerotic risk in Sprague–Dawley rats. Nutrients. 2017;9:766 https://doi.org/10.3390/nu9070766.
Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74:1251–62. https://doi.org/10.1038/s41430-020-0607-6.
Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715 https://doi.org/10.3390/microorganisms8111715.
Rivera-Piza A, Lee SJ. Effects of dietary fibers and prebiotics in adiposity regulation via modulation of gut microbiota. Appl Biol Chem. 2020;63:1–2. https://doi.org/10.1186/s13765-019-0482-9.
Xu Z, Jiang W, Huang W, Lin Y, Chan FK, Ng SC. Gut microbiota in patients with obesity and metabolic disorders—a systematic review. Genes Nutr. 2022;17:2 https://doi.org/10.1186/s12263-021-00703-6.
Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE. 2011;6:e20944 https://doi.org/10.1371/journal.pone.0020944.
Li H, Liu F, Lu J, Shi J, Guan J, Yan F, et al. Probiotic mixture of Lactobacillus plantarum strains improves lipid metabolism and gut microbiota structure in high-fat diet-fed mice. Front Microbiol. 2020;11:512. https://doi.org/10.3389/fmicb.2020.00512.
Ordiz MI, May TD, Mihindukulasuriya K, Martin J, Crowley J, Tarr PI, et al. The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children. Microbiome. 2015;3:1–9. https://doi.org/10.1186/s40168-015-0102-9.
Acknowledgements
Authors are thankful to the School of Science and the School of Pharmacy of RK University for providing the resources and support needed for smooth conduction of the study. First author is also highly thankful towards the Animal Research Facility, Zydus Research Centre, Ahmedabad, for providing the experimental animals. First author is also thankful toward the Govt. of Gujarat for providing the SHODH fellowship (KCG/SHODH/2022-23/202001600004). We also thank Dr. Jigna Kalaria (MD. Pathologist) and Dr. Vishal Bhatt (Chief Operating Officer), Pathology Lab Department of BT Savani Kidney Hospital, Rajkot for conducting histopathology study of animal organs and providing insightful interpretation. I would like to extend my heartfelt thanks to the Gujarat Biotechnology Research Center (GBRC) Gandhinagar for their support in the analysis of the metagenomic study. Special thanks to Ms. Khalida Bloch, Research Scholar, Department of Microbiology, School of Science, RK University, for her invaluable support during the in-vivo study.
Funding
The Govt. of Gujarat, India provided funds under their student startup and innovation policy (SSIP; reference no. RKU/SOS/SSIP/2023-2024/03) that was utilized for procurement of biochemical analysis kits, outsourcing of histopathological analysis of animal organs and metagenomic analysis of fecal samples. Beyond this, no other funding support has been received for this complete study.
Author information
Authors and Affiliations
Contributions
MC: Conceptualization, data Curation, investigation, methodology, visualization, writing—original draft, writing—review and editing. HM: Investigation, methodology. PM: Methodology. RN: Formal analysis, resources. PT: Resources. VK: Conceptualization, data curation, formal analysis, project administration, supervision, validation, visualization, writing-review and editing. All authors approved the final submission to the International Journal of Obesity.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study was approved by the Institutional Animal Ethics Committee (IAEC) of the School of Pharmacy, RK University, Rajkot, Gujarat, India (Proposal No. RKCP/Col/Re/22/131).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chauhan, M., Maniya, H., Mori, P. et al. Assessment of multi-strain probiotics in regulating diet-induced obesity in Balb/c mice model. Int J Obes 50, 202–212 (2026). https://doi.org/10.1038/s41366-025-01928-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01928-w