Abstract
Background
Obesity is a major health concern worldwide. Previous studies have suggested that phthalate plasticizers are obesogens. However, the relationship between early-life phthalate exposure and long-term obesity development remains unknown.
Objective
We investigated the association between prenatal phthalate exposure and children’s body mass index (BMI) patterns in an 18-year birth cohort follow-up study in Taiwan.
Methods
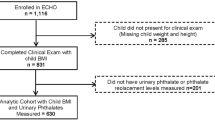
Our analytical lab quantified seven phthalate metabolites in maternal urine during pregnancy using quantitative liquid chromatography-tandem mass spectrometry. In addition, we calculated BMI z scores for participated children at each follow-up, utilized trajectory analysis to describe children’s BMI z-score patterns at 2–18 years of age, and adopted generalized estimating equations (GEE) and multivariate logistic regression models to assess the association between prenatal phthalate exposure and BMI z scores in children.
Results
A total of 208 mother-child pairs were included in the analysis. Maternal urinary diethyl phthalate (DEP) metabolites were associated with the increase of BMI z scores in children aged 2–18 years in the GEE model. Doubled maternal urinary ∑mDEHP (3 mono hexyl-metabolites of di-ethyl-hexyl phthalate (DEHP) increased the risk of children being in the stable-high BMI trajectory group until the age of eighteen.
Impact statement
We observed that BMI trajectories of children remained stable after the age of 5 years. During each follow-up, a higher frequency of overweight or obese was observed in children, ranging from 15.9% to 35.6% for girls and 15.2–32.0% for boys, respectively. Prenatal phthalate exposure was associated with increasing BMI z scores in children. Prenatal DEHP exposure was associated with a stable-high BMI trajectory in children up to the age of 18 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data analyzed during the current study are not publicly available due to sensitivity reasons. However, the data are available from the corresponding author on reasonable request. The data are securely stored at the National Health Research Institute, Taiwan.
References
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–49. https://doi.org/10.1016/S0140-6736(20)30752-2
Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–79. https://doi.org/10.1016/j.jaci.2018.02.004
Nehus E, Mitsnefes M. Childhood obesity and the metabolic syndrome. Pediatr Clin North Am. 2019;66:31–43. https://doi.org/10.1016/j.pcl.2018.08.004
Weihrauch-Bluher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–52. https://doi.org/10.1016/j.metabol.2018.12.001
WHO (World Health Organisation). Obesity and overweight, Fact sheet updated March 2024. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
Health Promotion Administration. Taiwan’s obesity prevention and management strategy. 2018. p. 56. Taipei, Taiwan: Author. [Chinese]. ISBN: 978-986-05-7935-2
Li YF, Lin SJ, Chiang TL. Timing of rapid weight gain and its effect on subsequent overweight or obesity in childhood: findings from a longitudinal birth cohort study. BMC Pediatr. 2020;20:293 https://doi.org/10.1186/s12887-020-02184-9
Jequier E. Pathways to obesity. Int J Obes Relat Metab Disord. 2002;26:S12–17. https://doi.org/10.1038/sj.ijo.0802123.
Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity. 2006;14:529–644. https://doi.org/10.1038/oby.2006.71
Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–61. https://doi.org/10.1038/nrendo.2015.163
Lanthier N, Leclercq IA. Adipose tissues as endocrine target organs. Best Pr Res Clin Gastroenterol. 2014;28:545–58. https://doi.org/10.1016/j.bpg.2014.07.002
Haverinen E, Fernandez MF, Mustieles V, Tolonen H. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health. 2021;18. https://doi.org/10.3390/ijerph182413047.
Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharm Toxicol. 2019;59:89–106. https://doi.org/10.1146/annurev-pharmtox-010818-021304
Yang C, Lee HK, Kong APS, Lim LL, Cai Z, Chung ACK. Early-life exposure to endocrine disrupting chemicals associates with childhood obesity. Ann Pediatr Endocrinol Metab. 2018;23:182–95. https://doi.org/10.6065/apem.2018.23.4.182
Ribeiro CM, Beserra BTS, Silva NG, Lima CL, Rocha PRS, Coelho MS, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10:e033509 https://doi.org/10.1136/bmjopen-2019-033509
Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–62. https://doi.org/10.1146/annurev-physiol-012110-142200
Lin LC, Wang SL, Chang YC, Huang PC, Cheng JT, Su PH, et al. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere. 2011;83:1192–9. https://doi.org/10.1016/j.chemosphere.2010.12.079
Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty'. Int J Epidemiol. 2016;45:1887–94. https://doi.org/10.1093/ije/dyw341
Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. https://doi.org/10.1146/annurev.clinpsy.121208.131413
Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–93. https://doi.org/10.1177/0049124101029003005
Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–76. https://doi.org/10.1152/physrev.00029.2013
Holtcamp W. Obesogens: an environmental link to obesity. Environ Health Perspect. 2012;120:a62–68. https://doi.org/10.1289/ehp.120-a62
Montes JOA, Villarreal AB, Romieu I, Barr DB, Martinez KC, Cadena LH. Modification of the association by sex between the prenatal exposure to di(2-ethylhexyl) phthalate and fat percentage in a cohort of Mexicans schoolchildren. Int J Obes. 2022;46:121–8. https://doi.org/10.1038/s41366-021-00952-w
Berger K, Hyland C, Ames JL, Mora AM, Huen K, Eskenazi B, et al. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in five-year-olds in the CHAMACOS cohort. Int J Environ Res Public Health. 2021;18. https://doi.org/10.3390/ijerph18041796.
Ferguson KK, Bommarito PA, Arogbokun O, Rosen EM, Keil AP, Zhao S, et al. Prenatal phthalate exposure and child weight and adiposity from in utero to 6 years of age. Environ Health Perspect. 2022;130:47006 https://doi.org/10.1289/EHP10077
Fan Y, Qin Y, Chen M, Li X, Wang R, Huang Z, et al. Prenatal low-dose DEHP exposure induces metabolic adaptation and obesity: Role of hepatic thiamine metabolism. J Hazard Mater. 2020;385:121534 https://doi.org/10.1016/j.jhazmat.2019.121534
Zhu YD, Gao H, Huang K, Zhang YW, Cai XX, Yao HY, et al. Prenatal phthalate exposure and placental size and shape at birth: a birth cohort study. Environ Res. 2018;160:239–46. https://doi.org/10.1016/j.envres.2017.09.012
Zong T, Lai L, Hu J, Guo M, Li M, Zhang L, et al. Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J Hazard Mater. 2015;297:25–33. https://doi.org/10.1016/j.jhazmat.2015.04.065
Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. https://doi.org/10.1093/toxsci/kfg145
Maradonna F, Carnevali O. Lipid metabolism alteration by endocrine disruptors in animal models: an overview. Front Endocrinol. 2018;9:654 https://doi.org/10.3389/fendo.2018.00654
Huang Y, Garcia JM, Shu W, Rong H, Zhang L, Wang Y, et al. Peroxisome proliferator activated receptor gamma in human placenta may mediate the adverse effects of phthalates exposure in pregnancy. Reprod Toxicol. 2018;75:121–6. https://doi.org/10.1016/j.reprotox.2017.10.001
Kratochvil I, Hofmann T, Rother S, Schlichting R, Moretti R, Scharnweber D, et al. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor gamma. Rapid Commun Mass Spectrom. 2019;33:75–85. https://doi.org/10.1002/rcm.8258.
Zeman FA, Boudet C, Tack K, Floch Barneaud A, Brochot C, Pery AR, et al. Exposure assessment of phthalates in French pregnant women: results of the ELFE pilot study. Int J Hyg Environ Health. 2013;216:271–9. https://doi.org/10.1016/j.ijheh.2012.12.005
Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, et al. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 2015;218:220–31. https://doi.org/10.1016/j.ijheh.2014.11.003
Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36:699–704. https://doi.org/10.1016/j.envint.2010.05.003
Zhu Y, Wan Y, Li Y, Zhang B, Zhou A, Cai Z, et al. Free and total urinary phthalate metabolite concentrations among pregnant women from the Healthy Baby Cohort (HBC), China. Environ Int. 2016;88:67–73. https://doi.org/10.1016/j.envint.2015.12.004
van den Dries MA, Ferguson KK, Keil AP, Pronk A, Spaan S, Ghassabian A, et al. Prenatal exposure to nonpersistent chemical mixtures and offspring IQ and emotional and behavioral problems. Environ Sci Technol. 2021;55:16502–14. https://doi.org/10.1021/acs.est.1c04455
Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, et al. Association of early life exposure to phthalates with obesity and cardiometabolic traits in childhood: sex specific associations. Front Public Health. 2018;6:327 https://doi.org/10.3389/fpubh.2018.00327
Preece AS, Shu H, Knutz M, Krais AM, Beko G, Bornehag CG. Indoor phthalate exposure and contributions to total intake among pregnant women in the SELMA study. Indoor Air. 2021;31:1495–508. https://doi.org/10.1111/ina.12813
Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–73. https://doi.org/10.1289/ehp.10749
Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–45. https://doi.org/10.1289/ehp.1104139
Rosen EM, Stevens DR, McNell EE, Wood ME, Engel SM, Keil AP, et al. Variability and longitudinal trajectories of phthalate and replacement biomarkers across pregnancy in the human placenta and phthalates study. Environ Sci Technol. 2023;57:13036–46. https://doi.org/10.1021/acs.est.3c04043
Wu CF, Chen HM, Sun CW, Chen ML, Hsieh CJ, Wang SL, et al. Cohort Profile: The Taiwan Maternal and Infant Cohort Study (TMICS) of phthalate exposure and health risk assessment. Int J Epidemiol. 2018;47:1047–1047j. https://doi.org/10.1093/ije/dyy067
Weaver VM, Kotchmar DJ, Fadrowski JJ, Silbergeld EK. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. 2016;26:1–8. https://doi.org/10.1038/jes.2015.8
Acknowledgements
We thank all the children and their mothers who participated in our 18-year follow-up study and the research team who supported the data collection, specimen collection, and data measurement.
Funding
This study was supported by the National Health Research Institutes (EM-109-PP-05, EM-111-PP-05, EM-113-PP-05) and the Ministry of Science and Technology (MOST 106-3114-B-400-002, MOST 108-2321-B-400-007), and National Science and Technology Council (NSC 112-2314-B-400-009), Taiwan.
Author information
Authors and Affiliations
Contributions
Wen carried out all statistical analyses, drafted the initial manuscript, interpreted the results, and critically reviewed and revised the manuscript; Su performed the clinical follow-up of children and collected the data; Sun and Tsai collected the data and performed experimental analyses; Wang conceptualized the study, coordinated and supervised data collection, interpreted the data, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, HJ., Su, PH., Sun, CW. et al. Maternal phthalate exposure and BMI trajectory in children—an 18-year birth cohort follow-up study. J Expo Sci Environ Epidemiol 34, 601–609 (2024). https://doi.org/10.1038/s41370-024-00696-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41370-024-00696-5