Abstract
Background
Plastic-related contaminants, such as bisphenols, can enter the maternal body and be transferred to breast milk. While common bisphenols such as bisphenol A, S, F and AF have been detected in previous studies, there is limited knowledge about the occurrence of other structurally similar compounds in human milk with potential endocrine-disrupting properties.
Objective
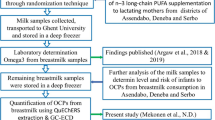
In this study, we investigated structural analogues and functional alternatives of bisphenol A (BPA) in 594 human milk samples collected from Canada (Montreal) and South Africa (Vhembe and Pretoria) using LC-Q-TOF-MS through suspect screening.
Methods
Suspect screening was performed using data collected from the milk samples using a customized database library (204 compounds). A retrospective semi-quantitative approach was then applied to estimate the levels of TGSA, D-8 and D-90 in human milk.
Results
This work revealed the presence of eleven compounds, including four compounds commonly used in thermal labels, four ultraviolet filters, and three synthetic antioxidants or metabolites. Retrospective semi-quantification of D-8, D-90 and TGSA revealed levels of up to 1.24, 1.98, and 0.72 ng/mL in milk, respectively.
Impact statement
Several structural analogues and functional alternatives of bisphenol A were identified in human milk through non-targeted screening. Two other phenolic compounds (Irganox 1010 and BHT-COOH) were identified in human milk for the first time. This study highlights the importance of novel strategies in human milk biomonitoring to identify emerging contaminants to which breastfeeding infants are exposed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data will be made available on request.
References
Martin CR, Ling P-R, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients, 2016;8:279. https://doi.org/10.3390/nu8050279.
Boquien C-Y. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front Pediatr. 2018;6. https://doi.org/10.3389/fped.2018.00295.
Wiciński M, Sawicka E, Gębalski J, Kubiak K, Malinowski B. Human milk oligosaccharides: health benefits, potential applications in infant formulas, and pharmacology. Nutrients. 2020;12:266.
Chi ZH, Goodyer CG, Hales BF, Bayen S. Characterization of different contaminants and current knowledge for defining chemical mixtures in human milk: A review. Environ Int. 2023;171:107717. https://doi.org/10.1016/j.envint.2022.107717.
Tran CD, Dodder NG, Quintana PJE, Watanabe K, Kim JH, Hovell MF. et al. Organic contaminants in human breast milk identified by non-targeted analysis. Chemosphere. 2020;238:124677. https://doi.org/10.1016/j.chemosphere.2019.124677.
Kunzelmann M, Winter M, Åberg M, Hellenäs K-E, Rosén J. Non-targeted analysis of unexpected food contaminants using LC-HRMS. Anal Bioanal Chem. 2018;410:5593–602. https://doi.org/10.1007/s00216-018-1028-4.
Manz KE, Feerick A, Braun JM, Feng Y-L, Hall A, Koelmel J. et al. Non-targeted analysis (NTA) and suspect screening analysis (SSA): a review of examining the chemical exposome. J Expos Sci Environ Epidemiol. 2023;33:524–36. https://doi.org/10.1038/s41370-023-00574-6.
Place BJ, Ulrich EM, Challis JK, Chao A, Du B, Favela K. et al. An introduction to the benchmarking and publications for non-targeted analysis working group. Anal Chem. 2021;93:16289–96. https://doi.org/10.1021/acs.analchem.1c02660.
Mukherjee T, Kumar V, Pal S. Chapter 1—Sampling and analysis of emerging pollutants in aquatic environment. In: Kumar M, Mohapatra S, Weber, K, editors. Emerging Aquatic Contaminants. Elsevier, Amsterdam, Netherlands; 2023. p. 3–34.
Pourchet M, Narduzzi L, Jean A, Guiffard I, Bichon E, Cariou R. et al. Non-targeted screening methodology to characterise human internal chemical exposure: Application to halogenated compounds in human milk. Talanta. 2021;225:121979. https://doi.org/10.1016/j.talanta.2020.121979.
Musatadi M, Andrés-Maguregi A, De Angelis F, Prieto A, Anakabe E, Olivares M. et al. The role of sample preparation in suspect and non-target screening for exposome analysis using human urine. Chemosphere. 2023;339:139690. https://doi.org/10.1016/j.chemosphere.2023.139690.
Musatadi M, Gonzalez-Gaya B, Irazola M, Prieto A, Etxebarria N, Olivares M. et al. Multi-target analysis and suspect screening of xenobiotics in milk by UHPLC-HRMS/MS. Separations. 2021;8:14. https://doi.org/10.3390/separations8020014.
Zheng S, Wang J, Luo K, Gu X, Yuan G, Wei M. et al. Comprehensive characterization of organic light-emitting materials in breast milk by target and suspect screening. Environ Sci Technol. 2024;58:5103–16. https://doi.org/10.1021/acs.est.3c08961.
Abdulhameed AAR, Lim V, Bahari H, Khoo BY, Abdullah MNH, Tan JJ, et al. Adverse effects of bisphenol a on the liver and its underlying mechanisms: evidence from in vivo and in vitro studies. Biomed Res Int. 2022;2022:8227314 https://doi.org/10.1155/2022/8227314.
Ahern TP, Spector LG, Damkier P, Öztürk Esen B, Ulrichsen SP, Eriksen K, et al. Medication–associated phthalate exposure and childhood cancer incidence. J Natl Cancer Inst. 2022;114:885–94. https://doi.org/10.1093/jnci/djac045.
Alharbi HF, Algonaiman R, Alduwayghiri R, Aljutaily T, Algheshairy RM, Almutairi AS, et al. Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review. Int J Environ Res Public Health. 2022, 19. https://doi.org/10.3390/ijerph192315918.
Brucker-Davis F, Wagner-Mahler K, Bornebusch L, Delattre I, Ferrari P, Gal J. et al. Exposure to selected endocrine disruptors and neonatal outcome of 86 healthy boys from Nice area (France). Chemosphere. 2010;81:169–76. https://doi.org/10.1016/j.chemosphere.2010.06.068.
Chi ZH, Liu L, Zheng J, Tian L, Chevrier J, Bornman R. et al. Biomonitoring of bisphenol A (BPA) and bisphenol analogues in human milk from South Africa and Canada using a modified QuEChERS extraction method. Environ Pollut. 2024;348:123730. https://doi.org/10.1016/j.envpol.2024.123730.
Health Canada. Technical consultation: proposed subgrouping of bisphenol A (BPA) structural analogues and functional alternatives. Health Canada, Ed.; 2020.
Luo D, Pan Y, Zeng L, Du B, Li J, Mei S. Occurrence of multiple Bisphenol S derivatives in breast milk from Chinese lactating women and implications for exposure in breast-fed infants. Environ Sci Technol Lett. 2021;8:176–82. https://doi.org/10.1021/acs.estlett.0c00883.
den Braver-Sewradj SP, van Spronsen R, Hessel EVS. Substitution of bisphenol A: a review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit Rev Toxicol. 2020;50:128–47. https://doi.org/10.1080/10408444.2019.1701986.
Kim B, Kwon B, Jang S, Kim P-G, Ji K. Major benzophenone concentrations and influence of food consumption among the general population in Korea, and the association with oxidative stress biomarker. Sci Total Environ. 2016;565:649–55. https://doi.org/10.1016/j.scitotenv.2016.05.009.
Anderson WAC, Castle L. Benzophenone in cartonboard packaging materials and the factors that influence its migration into food. Food Addit Contam. 2003;20:607–18. https://doi.org/10.1080/0265203031000109486.
Wang L, Asimakopoulos AG, Moon H-B, Nakata H, Kannan K. Benzotriazole, Benzothiazole, and Benzophenone compounds in indoor dust from the United States and East Asian countries. Environ Sci Technol. 2013;47:4752–9. https://doi.org/10.1021/es305000d.
Tian L, Zheng J, Pineda M, Yargeau V, Furlong D, Chevrier J. et al. Targeted screening of 11 bisphenols and 7 plasticizers in food composites from Canada and South Africa. Food Chem. 2022;385:132675. https://doi.org/10.1016/j.foodchem.2022.132675.
Nerı́n C, Philo MR, Salafranca J, Castle L. Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction–high-performance liquid chromatography. J Chromatogr A. 2002;963:375–80. https://doi.org/10.1016/S0021-9673(02)00554-X.
Yang Y, Guan J, Yin J, Shao B, Li H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere. 2014;112:481–6. https://doi.org/10.1016/j.chemosphere.2014.05.004.
Vasiljevic T, Harner T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci Total Environ. 2021;789:148013. https://doi.org/10.1016/j.scitotenv.2021.148013.
Baloul H, Belhaneche-Bensemra N, Quirós ARBD, Sendon R. Analysis and quantitative estimation of phenolic antioxidants in polypropylene packaging for fat products. J Polym Eng. 2018;38:899–904. https://doi.org/10.1515/polyeng-2017-0454.
Cao X-L, Popovic S, Arbuckle TE, Fraser WD. Determination of free and total bisphenol A in human milk samples from Canadian women using a sensitive and selective GC-MS method. Food Addit Contam: Part A. 2015;32:120–5. https://doi.org/10.1080/19440049.2014.980855.
Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching–high performance liquid chromatography–isotope dilution tandem mass spectrometry. J Chromatogr B. 2006;831:110–5. https://doi.org/10.1016/j.jchromb.2005.11.050.
Dualde P, Pardo O, Corpas-Burgos F, Kuligowski J, Gormaz M, Vento M. et al. Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci Total Environ. 2019;668:797–805. https://doi.org/10.1016/j.scitotenv.2019.03.024.
Jin H, Xie J, Mao L, Zhao M, Bai X, Wen J. et al. Bisphenol analogue concentrations in human breast milk and their associations with postnatal infant growth. Environ Pollut. 2020;259:113779. https://doi.org/10.1016/j.envpol.2019.113779.
Lee J, Choi K, Park J, Moon H-B, Choi G, Lee JJ. et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Sci Total Environ. 2018;626:1494–501. https://doi.org/10.1016/j.scitotenv.2017.10.042.
Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622:150–6. https://doi.org/10.1016/j.aca.2008.05.068.
Deceuninck Y, Bichon E, Marchand P, Boquien C-Y, Legrand A, Boscher C. et al. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407:2485–97. https://doi.org/10.1007/s00216-015-8469-9.
Samanidou VF, Frysali MA, Papadoyannis IN. Matrix solid phase dispersion for the extraction of bisphenol-a from human breast milk prior to HPLC analysis. J Liq Chromatogr Relat Technol. 2014;37:247–58. https://doi.org/10.1080/10826076.2012.745133.
Goldinger DM, Demierre A-L, Zoller O, Rupp H, Reinhard H, Magnin R. et al. Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul Toxicol Pharmacol. 2015;71:453–62. https://doi.org/10.1016/j.yrtph.2015.01.002.
Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. https://doi.org/10.1016/j.jsbmb.2011.05.002.
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K. et al. Comparative study of the endocrine-disrupting activity of Bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–59. https://doi.org/10.1093/toxsci/kfi074.
Ng HW, Shu M, Luo H, Ye H, Ge W, Perkins R. et al. Estrogenic activity data extraction and in silico prediction show the endocrine disruption potential of Bisphenol A replacement compounds. Chem Res Toxicol. 2015;28:1784–95. https://doi.org/10.1021/acs.chemrestox.5b00243.
McCord JP, Groff LC, Sobus JR. Quantitative non-targeted analysis: Bridging the gap between contaminant discovery and risk characterization. Environ Int. 2022;158:107011. https://doi.org/10.1016/j.envint.2021.107011.
Malm L, Palm E, Souihi A, Plassmann, M, Liigand J, Kruve A. Guide to semi-quantitative non-targeted screening using LC/ESI/HRMS. Molecules 2021;26. https://doi.org/10.3390/molecules26123524.
Wang W, Kannan K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ Int. 2019;128:24–29. https://doi.org/10.1016/j.envint.2019.04.028.
Zhang Y, Du B, Ge J, Liu L, Zhu M, Li J. et al. Co-occurrence of and infant exposure to multiple common and unusual phenolic antioxidants in human breast milk. Environ Sci Technol Lett. 2020;7:206–12. https://doi.org/10.1021/acs.estlett.0c00104.
Rodríguez-Gómez R, Zafra-Gómez A, Dorival-García N, Ballesteros O, Navalón A. Determination of benzophenone-UV filters in human milk samples using ultrasound-assisted extraction and clean-up with dispersive sorbents followed by UHPLC–MS/MS analysis. Talanta. 2015;134:657–64. https://doi.org/10.1016/j.talanta.2014.12.004.
Pouech C, Kiss A, Lafay F, Léonard D, Wiest L, Cren-Olivé C. et al. Human exposure assessment to a large set of polymer additives through the analysis of urine by solid phase extraction followed by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A. 2015;1423:111–23. https://doi.org/10.1016/j.chroma.2015.10.091.
Peter KT, Phillips AL, Knolhoff AM, Gardinali PR, Manzano CA, Miller KE. et al. Nontargeted analysis study reporting tool: a framework to improve research transparency and Reproducibility. Anal Chem. 2021;93:13870–9. https://doi.org/10.1021/acs.analchem.1c02621.
Nason SL, Koelmel J, Zuverza-Mena N, Stanley C, Tamez C, Bowden JA. et al. Software comparison for nontargeted analysis of PFAS in AFFF-contaminated soil. J Am Soc Mass Spectrom. 2021;32:840–6. https://doi.org/10.1021/jasms.0c00261.
Dührkop K, Fleischauer M, Ludwig M, Aksenov AA, Melnik AV, Meusel M. et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods. 2019;16:299–302. https://doi.org/10.1038/s41592-019-0344-8.
SANTE/11312/2021. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed. 2021.
Diana Di Mavungu J, Monbaliu S, Scippo M-L, Maghuin-Rogister G, Schneider Y-J, Larondelle Y. et al. LC-MS/MS multi-analyte method for mycotoxin determination in food supplements. Food Addit Contam A Chem Anal Control Exposure Risk Assess. 2009;26:885–95. https://doi.org/10.1080/02652030902774649.
Rezk MR, Basalious EB, Karim IA. Development of a sensitive UPLC-ESI-MS/MS method for quantification of sofosbuvir and its metabolite, GS-331007, in human plasma: Application to a bioequivalence study. J Pharm Biomed Anal. 2015;114:97–104. https://doi.org/10.1016/j.jpba.2015.05.006.
EPA, U. S. E.P.A. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2. EPA, Ed.; Office of Water, 2016; Vol. EPA 821-R-16-006.
Rice EW, Bridgewater L, Association, APH Standard methods for the examination of water and wastewater. Washington, DC; American Public Health Association: 2012.
von Eyken A, Furlong D, Arooni S, Butterworth F, Roy J-F, Zweigenbaum J. et al. Direct injection high performance liquid chromatography coupled to data independent acquisition mass spectrometry for the screening of antibiotics in honey. J Food Drug Anal. 2019;27:679–91. https://doi.org/10.1016/j.jfda.2018.12.013.
Schymanski EL, Singer HP, Slobodnik J, Ipolyi IM, Oswald P, Krauss M. et al. Non-target screening with high-resolution mass spectrometry: critical review using a collaborative trial on water analysis. Anal Bioanal Chem. 2015;407:6237–55. https://doi.org/10.1007/s00216-015-8681-7.
Sewwandi M, Wijesekara H, Rajapaksha AU, Soysa S, Vithanage M. Microplastics and plastics-associated contaminants in food and beverages; Global trends, concentrations, and human exposure. Environ Pollut. 2023;317:120747. https://doi.org/10.1016/j.envpol.2022.120747.
Chen D, Kannan K, Tan H, Zheng Z, Feng Y-L, Wu Y. et al. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, And Toxicity—a Review. Environ Sci Technol. 2016;50:5438–53. https://doi.org/10.1021/acs.est.5b05387.
Lehmler H-J, Liu B, Gadogbe M, Bao W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults And Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega. 2018;3:6523–32. https://doi.org/10.1021/acsomega.8b00824.
Ji Z, Liu J, Sakkiah S, Guo W, Hong H. BPA replacement compounds: current status and perspectives. ACS Sustain Chem Eng. 2021;9:2433–46. https://doi.org/10.1021/acssuschemeng.0c09276.
U.S. Food and Drug Administration. Bisphenol A (BPA): Use in Food Contact Application. FDA, Ed.; 2014.
Erler C, Novak J. Bisphenol a exposure: human risk and health policy. J Pediatr Nurs. 2010;25:400–7. https://doi.org/10.1016/j.pedn.2009.05.006.
Eckardt M, Simat TJ. Bisphenol A and alternatives in thermal paper receipts - a German market analysis from 2015 to 2017. Chemosphere. 2017;186:1016–25. https://doi.org/10.1016/j.chemosphere.2017.08.037.
Xu Z, Tian L, Liu L, Goodyer CG, Hales BF, Bayen S. Food thermal labels are a source of dietary exposure to bisphenol s and other color developers. Environ Sci Technol. 2023;57:4984–91. https://doi.org/10.1021/acs.est.2c09390.
Pelch KE, Li Y, Perera L, Thayer KA, Korach KS. Characterization of estrogenic and androgenic activities for Bisphenol A-like Chemicals (BPs): In vitro estrogen and androgen receptors transcriptional activation, gene regulation, and binding profiles. Toxicol Sci. 2019;172:23–37. https://doi.org/10.1093/toxsci/kfz173.
Pan Y, Zhu J, Zhu Z, Wei X, Zhou X, Yin R, et al. Occurrence of multiple bisphenol S analogues in children from Shantou, China. Environ Int. 2023;174:107926. https://doi.org/10.1016/j.envint.2023.107926
Iribarne-Durán LM, Peinado FM, Freire C, Castillero-Rosales I, Artacho-Cordón F, Olea N. Concentrations of bisphenols, parabens, and benzophenones in human breast milk: A systematic review and meta-analysis. Sci Total Environ. 2022;806:150437. https://doi.org/10.1016/j.scitotenv.2021.150437.
Rodríguez-Gómez R, Jiménez-Díaz I, Zafra-Gómez A, Ballesteros O, Navalón A. A multiresidue method for the determination of selected endocrine disrupting chemicals in human breast milk based on a simple extraction procedure. Talanta. 2014;130:561–70. https://doi.org/10.1016/j.talanta.2014.07.047.
Molins-Delgado D, Olmo-Campos MdM, Valeta-Juan G, Pleguezuelos-Hernández V, Barceló D, Díaz-Cruz MS. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ Res. 2018;161:532–9. https://doi.org/10.1016/j.envres.2017.11.033.
Iribarne-Durán LM, Serrano L, Peinado FM, Peña-Caballero M, Hurtado JA, Vela-Soria F. et al. Biomonitoring bisphenols, parabens, and benzophenones in breast milk from a human milk bank in Southern Spain. Sci Total Environ. 2022;830:154737. https://doi.org/10.1016/j.scitotenv.2022.154737.
Mao JF, Li W, Ong CN, He Y, Jong M-C, Gin KY-H. Assessment of human exposure to benzophenone-type UV filters: A review. Environ Int. 2022;167:107405. https://doi.org/10.1016/j.envint.2022.107405
Hu L, Tian M, Feng W, He H, Wang Y, Yang L. Sensitive detection of benzophenone-type ultraviolet filters in plastic food packaging materials by sheathless capillary electrophoresis–electrospray ionization–tandem mass spectrometry. J Chromatogr A. 2019;1604:460469. https://doi.org/10.1016/j.chroma.2019.460469.
Medina-Pérez NI, Arrizabalaga-Larrañaga A, Seró R, Moyano E. Determination of benzophenone and related compounds in plastic packaged baby food by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Anal Methods. 2020;12:358–67.
Snedeker SM. Benzophenone UV-Photoinitiators Used in Food Packaging: Potential for Human Exposure and Health Risk Considerations. In: Snedeker SM editor. Toxicants in Food Packaging and Household Plastics: Exposure and Health Risks to Consumers. London: Springer; 2014. p. 151-76.
Lee J, Kim S, Park YJ, Moon H-B, Choi K. Thyroid hormone-disrupting potentials of major benzophenones in two cell lines (GH3 and FRTL-5) and embryo-larval Zebrafish. Environ Sci Technol. 2018;52:8858–65. https://doi.org/10.1021/acs.est.8b01796.
Xu M, Zheng D, Gong S. Effects of low concentration benzophenone-3 exposure on the sex ratio and offspring development of Zebrafish (Danio rerio). Bull Environ Contamin Toxicol. 2021;106:740–6. https://doi.org/10.1007/s00128-021-03166-y.
Yao Y-N, Wang Y, Zhang H, Gao Y, Zhang T, Kannan K. A review of sources, pathways, and toxic effects of human exposure to benzophenone ultraviolet light filters. Eco-Environ Health. 2024;3:30–44. https://doi.org/10.1016/j.eehl.2023.10.001.
Kang K, Chang Y, Choi JC, Park S-J, Han J. Migration Study of Butylated Hydroxytoluene and Irganox 1010 from polypropylene treated with severe processing conditions. J Food Sci. 2018;83:1005–10. https://doi.org/10.1111/1750-3841.14104.
Zabihzadeh Khajavi M, Ahmadi S, Abedi A-S, Mohammadi R, Farhoodi M. A model study on the migration of Irganox 1010 from low-density polyethylene into a fatty food simulant as a function of incorporated spherical and plate-like nanoparticles. Food Packag Shelf Life. 2019;22:100333. https://doi.org/10.1016/j.fpsl.2019.100333.
Tian L, Zheng J, Goodyer CG, Bayen S. Non-targeted screening of plastic-related chemicals in food collected in Montreal, Canada. Food Chem. 2020;326:126942. https://doi.org/10.1016/j.foodchem.2020.126942.
Dopico-García MS, López-Vilariñó JM, González-Rodríguez MV. Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages. J Agric Food Chem. 2007;55:3225–31. https://doi.org/10.1021/jf070102.
Gao Y, Gu Y, Wei Y. Determination of polymer additives–antioxidants and ultraviolet (UV) absorbers by high-performance liquid chromatography coupled with UV Photodiode array detection in food simulants. J Agric Food Chem. 2011;59:12982–9. https://doi.org/10.1021/jf203257b.
Hattaway ME, Black GP, Young TM. Batch correction methods for nontarget chemical analysis data: application to a municipal wastewater collection system. Anal Bioanal Chem. 2023;415:1321–31. https://doi.org/10.1007/s00216-023-04511-2.
Nilsson LB, Eklund G. Direct quantification in bioanalytical LC–MS/MS using internal calibration via analyte/stable isotope ratio. J Pharm Biomed Anal. 2007;43:1094–9. https://doi.org/10.1016/j.jpba.2006.09.030.
Steiner D, Krska R, Malachová A, Taschl I, Sulyok M. Evaluation of Matrix effects and extraction efficiencies of LC-MS/MS methods as the essential part for proper validation of multiclass contaminants in complex feed. J Agric Food Chem. 2020;68:3868–80. https://doi.org/10.1021/acs.jafc.9b07706.
Kellogg JJ, Graf TN, Paine MF, McCune JS, Kvalheim OM, Oberlies NH. et al. Comparison of metabolomics approaches for evaluating the variability of complex botanical preparations: green tea (Camellia sinensis) as a case study. J Nat Products. 2017;80:1457–66. https://doi.org/10.1021/acs.jnatprod.6b01156.
Stolker AAM, Niesing W, Fuchs R, Vreeken RJ, Niessen WMA, Brinkman UAT. Liquid chromatography with triple-quadrupole and quadrupole-time-of-flight mass spectrometry for the determination of micro-constituents – a comparison. Anal Bioanal Chem. 2004;378:1754–61. https://doi.org/10.1007/s00216-003-2485-x.
Lambré C, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023;21:e06857. https://doi.org/10.2903/j.efsa.2023.6857.
Wang X, Nag R, Brunton NP, Siddique MAB, Harrison SM, Monahan FJ. et al. Human health risk assessment of bisphenol A (BPA) through meat products. Environ Res. 2022;213:113734. https://doi.org/10.1016/j.envres.2022.113734.
Björnsdotter MK, de Boer J, Ballesteros-Gómez A. Bisphenol A and replacements in thermal paper: A review. Chemosphere. 2017;182:691–706. https://doi.org/10.1016/j.chemosphere.2017.05.070.
Li K, Bertrand K, Naviaux JC, Monk JM, Wells A, Wang L. et al. Metabolomic and exposomic biomarkers of risk of future neurodevelopmental delay in human milk. Pediatr Res. 2023;93:1710–20. https://doi.org/10.1038/s41390-022-02283-6.
Krausová M, Braun D, Buerki-Thurnherr T, Gundacker C, Schernhammer E, Wisgrill L. et al. Understanding the chemical exposome during fetal development and early childhood: a review. Annu Rev Pharmacol Toxicol. 2023;63:517–40. https://doi.org/10.1146/annurev-pharmtox-051922-113350.
Acknowledgements
We wish to acknowledge the financial support received from the Canadian Institutes of Health Research (CIHR) (IP3-150711: Endocrine disrupting chemicals: towards responsible replacements; Principal Investigators: B. F. Hales; B. Robaire), the Canada Foundation for Innovation/John R. Evans Leaders Fund grant (Project #35318; S. Bayen) and a Canada Research Chair in Global Environmental Health and Epidemiology held by J. Chevrier).
Author information
Authors and Affiliations
Contributions
Zhi Hao Chi: Writing—review and editing, Writing—original draft, Methodology, Formal analysis, Data curation, Conceptualization. Lan Liu: Writing—review and editing, Formal analysis. Jingyun Zheng: Writing—review and editing, Formal analysis. Lei Tian: Writing—review and editing, Formal analysis. Jonathan Chevrier: Writing—review and editing, Resources, Methodology, Funding acquisition. Riana Bornman: Writing—review and editing, Project administration, Methodology, Funding acquisition. Muvhulawa Obida: Writing—review and editing, Methodology, Investigation, Funding acquisition. Cindy Gates Goodyer: Writing—review and editing, Methodology, Funding acquisition, Conceptualization. Barbara F. Hales: Writing—review and editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition. Stéphane Bayen: Writing—review and editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chi, Z.H., Liu, L., Zheng, J. et al. Suspect screening of bisphenol A (BPA) structural analogues and functional alternatives in human milk from Canada and South Africa. J Expo Sci Environ Epidemiol 35, 557–566 (2025). https://doi.org/10.1038/s41370-025-00782-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41370-025-00782-2