Abstract
Objective
To evaluate whether premature infants delivered ≤7 days after rescue antenatal steroid treatment (ideal treatment) have increased passive respiratory compliance compared to those delivered >7 days after treatment (remote treatment).
Methods
Secondary analysis of a randomized trial of rescue antenatal steroids on respiratory compliance. Infants in the treatment group were stratified by the interval between rescue antenatal steroids and delivery. We then compared the respiratory compliance in the ideal vs. remote groups.
Results
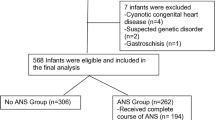
Forty-four women (56 infants) received rescue antenatal steroids. Forty-nine infants had evaluable respiratory compliance measurements, with 27 (GA 30.1 weeks, BW 1362 g) “ideally” treated, and 22 (GA 33.8 weeks, BW 2248 g) “remotely” treated. Respiratory compliance was significantly higher for the ideal compared to the remote group (1.32 vs. 1.06 mL/cm H2O/kg; p = 0.037).
Conclusion
Infants treated with rescue antenatal steroids have a significantly higher respiratory compliance if delivery occurs within 7 days after treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25.
Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–84.
McEvoy C, Bowling S, Williamson K, Stewart M, Durand M. Functional residual capacity and passive compliance measurements after antenatal steroid therapy in preterm infants. Pediatr Pulmonol. 2001;31:425–30.
Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454.
Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD. Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. Am J Respir Crit Care Med. 2001;163:1437–43.
Bunton TE, Plopper CG. Triamcinolone-induced structural alterations in the development of the lung of the fetal rhesus macaque. Am J Obstet Gynecol. 1984;148:203–15.
Johnson JW, Mitzner W, Beck JC, London WT, Sly DL, Lee PA, et al. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol. 1981;141:1053–64.
Tan RC, Ikegami M, Jobe AH, Yao LY, Possmayer F, Ballard PL. Developmental and glucocorticoid regulation of surfactant protein mRNAs in preterm lambs. Am J Physiol. 1999;277:L1142–48.
McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Aust N Z J Obstet Gynaecol. 2003;43:101–6.
McEvoy C, Schilling D, Spitale P, Peters D, O’Malley J, Durand M. Decreased respiratory compliance in infants less than or equal to 32 weeks’ gestation, delivered more than 7 days after antenatal steroid therapy. Pediatrics. 2008;121:e1032–38.
French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21.
Newnham JP, Simmer K. Multiple courses of antenatal corticosteroids. Lancet. 2008;372:2094–5.
Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–42.
Committee Opinion No. 713. Summary: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:493–4.
McEvoy C, Schilling D, Peters D, Tillotson C, Spitale P, Wallen L, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. Am J Obstet Gynecol. 2010;202:544.e1–9.
Crowther CA, McKinlay CJD, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2015; CD003935.
Bates JH, Schmalisch G, Filbrun D, Stocks J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1180–92.
Gappa M, Colin AA, Goetz I, Stocks J. ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Passive respiratory mechanics: the occlusion techniques. Eur Respir J. 2001;17:141–8.
Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J. ERS/ATS task force on standards for infant respiratory function testing. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2001;17:529–36.
LeSouef PN, England SJ, Bryan AC. Passive respiratory mechanics in newborns and children. Am Rev Respir Dis. 1984;129:552–6.
Håland G, Carlsen KCL, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–9.
NIH Consens Statement Online. The effect of antenatal steroids for fetal maturation on perinatal outcomes-interim draft statement. 1994; 12: 1–24.
Abbasi S, Hirsch D, Davis J, Tolosa J, Stouffer N, Debbs R, et al. Effect of single versus multiple courses of antenatal corticosteroids on maternal and neonatal outcome. Am J Obstet Gynecol. 2000;182:1243–9.
Peaceman AM, Bajaj K, Kumar P, Grobman WA. The interval between a single course of antenatal steroids and delivery and its association with neonatal outcomes. Am J Obstet Gynecol. 2005;193:1165–9.
Garite TJ, Kurtzman J, Maurel K, Clark R. Obstetrix collaborative research network. Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248.e1–9.
Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–51.
Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol. 1996;14:599–607.
Goldenberg RL, McClure EM. Appropriate use of antenatal corticosteroid prophylaxis. Obstet Gynecol. 2015;125:285–7.
Funding
This work was supported by National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000128. NIH, National Heart Lung Blood Institute, K23 HL080231 and R01 HL105447 with co-funding from the Office of Dietary Supplement; and American Lung Association (CTM). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jordan, B.K., Schilling, D. & McEvoy, C.T. The window of improved neonatal respiratory compliance after rescue antenatal steroids. J Perinatol 38, 828–833 (2018). https://doi.org/10.1038/s41372-018-0124-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-018-0124-9