Abstract
Objective
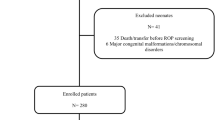
To systematically assess the efficacy of oral beta blockage treatment in primary (before established) and secondary (in threshold stages) prevention of severe retinopathy of prematurity (ROP) in premature infants born ≤32 weeks gestational age.
Study design
Following the PRISMA guidelines, published literature was systematically assessed up to April 27, 2018. Trials and observational studies, in which beta blockage was used to prevent severe ROP (defined as stage ≥3, or requiring treatment) were included. Meta-analyses including random effects models were conducted to determine the overall effect of oral beta blockage on prevention of ROP.
Results
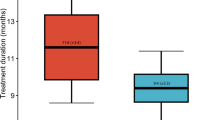
Six studies (five clinical trials and one observational study) including 461 infants met inclusion criteria using propranolol. The pooled relative risk (RR) of severe ROP in the primary and secondary prophylaxis groups were 0.65 (95% CI 0.43–0.98, NNT = 7) and 0.48 (95% CI 0.35–0.65, NNT = 6) in RCTs, respectively. The RR of severe ROP in one observational study was 0.21 (95% CI 0.08–0.55) with a NNT of 3. There were low heterogeneity and publication bias. Side effects occurred in 8.4% of participants on propranolol.
Conclusions
Systematic assessment of studies showed that prophylactic oral propranolol appeared to be effective in preventing severe ROP in premature infants ≤32 weeks gestational age. Additional well powered, multinational, randomized control trials reporting on long-term outcomes are needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
29 October 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57.
Canadian Neonatal N. Annual Report. 2016. http://www.canadianneonatalnetworkorg/Portal/LinkClickaspx?fileticket=PJSDwNECsMI%3d&tabid=39. Accessed 15 Jan 2019.
Mutlu F, Sarici SU. Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic options. Int J Ophthalmol. 2013;6:228–36.
Early Treatment for ROP Cooperative, Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Opthalmol. 2003;121:1684–94.
Mintz-Hittner H, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Eng J Med. 2011;364:603–15.
Selvam S, Kumar T, Fruttiger M. Retinal vasculature development in health and disease. Prog Retin Eye Res. 2018;63:1–19.
Smith L. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):S140–4.
Pierce E, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Opthalmol. 1996;114:1219–28.
Young T, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J Aapos. 1997;1:105–10.
Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Eng J Med. 2015;372:735–46.
Ristori C, Filippi L, Dal Monte M, Martini D, Cammalleri M, Fortunato P, et al. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest Ophthalmol Vis Sci. 2011;52:155–70.
Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res. 2014;42:103–29.
Dal Monte M, Casini G, la Marca G, Isacchi B, Filippi L, Bagnoli P. Eye drop propranolol administration promotes the recovery of oxygen-induced retinopathy in mice. Exp Eye Res. 2013;111:27–35.
Chen J, Joyal JS, Hatton CJ, Juan AM, Pei DT, Hurst CG, et al. Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularizaion in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53;2968–77.
Hård A, Hellström A. On the use of antiangiogenetic medications for retinopathy of prematurity. Acta Paediatr. 2011;100:1063–5.
Kaempfen S, Neumann RP, Jost K, Schulzke SM. Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Cochrane Database Syst Rev. 2018;3:CD011893.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Bancalari A, Schade R, Lazcano C, Munoz T, Sepulveda G. Treatment of retinopathy of prematurity with propranolol: a randomized control trial. PAS-oral presentation. 2018.
Burns P, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–10.
International Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. ROB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:14898;1–8
Wells GA, Shea B, O'Connell D, Peterson J, Welche V, Losos M, et al. The Newcastle-Ottawa (NOC) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 15 Jan 2019.
Bancalari A, Schade R, Muñoz T, Lazcano C, Parada R, Pena R. Oral propranolol in early stages of retinopathy of prematurity. J Perinat Med. 2016;44:499–503.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Sanghvi K, Kabra NS, Padhi P, Singh U, Dash SK, Avasthi BS. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial). Arch Dis Child Fetal Neonatal Ed. 2017;102:F389–94.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Donzelli G, et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr. 2013;163:1570–7.
Makhoul I, Peleg O, Miller B, Bar-Oz B, Kochavi O, Mechoulam H, et al. Oral propranolol versus placebo for retinopathy of prematurity: a pilot, randomised, double-blind prospective study. Arch Dis Child. 2013;98:565–7.
Korkmaz L, Baştuğ O, Ozdemir A, Korkut S, Karaca C, Akin MA, et al. The efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Curr Eye Res. 2017;42:88–97.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Berti E, et al. Propranolol 0.1% eye micro-drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr Res. 2017;81:307–14.
Bremond-Gignac D, Jacqz-Aigrain E, Abdoul H, Daruich A, Beresniak A, Baud O, et al. on behalf of the CLAIR FO study group. Ophthalmic insert versus eye drops for Mydriasis in neonates: a randomized clinical trial. Neonatology. 2018;115:142–8.
Darlow B, Lui K, Kusuda S, Reichman B, Håkansson S, Bassler D, et al. International Network for Evaluating Outcomes of Neonates. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol. 2017;101:1399–404.
Acknowledgements
We would like to thank Dr Aldo Bancalari, Dr Filippi, Dr Sanghvi, and Dr Makhoul for providing extra data through email and phone conversations on their respective studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These finding were presented in an oral presentation at the Pediatric Academic Society Meeting 2018 in Toronto, and as poster presentation at the District VIII American Academy of Pediatrics Meeting 2018 in Utah.
Rights and permissions
About this article
Cite this article
Stritzke, A., Kabra, N., Kaur, S. et al. Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol 39, 1584–1594 (2019). https://doi.org/10.1038/s41372-019-0503-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-019-0503-x
This article is cited by
-
Retinopathy of prematurity in the era of precision neonatology: from risk stratification to targeted therapies
World Journal of Pediatrics (2025)
-
Comparison of Different Doses of Oral and Ocular Propranolol for Retinopathy of Prematurity: A Network Meta-Analysis
Pediatric Drugs (2024)
-
Comparative study on optic disc features of premature infants and full‐term newborns
BMC Ophthalmology (2021)