Abstract
Objective
To determine whether ventilator-related fluctuations in cerebral blood volume (CBV) are associated with cerebral pressure passivity.
Study design
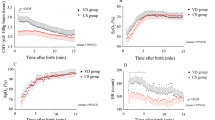
In a prospective study of newborns undergoing positive-pressure ventilation, we calculated coherence between continuous mean arterial pressure (MAP) and cerebral near-infrared spectroscopy hemoglobin difference (HbD). Significant HbD–MAP coherence indicated cerebral pressure passivity. CBV changes were measured as the spectral power of total hemoglobin (SHbT) at the ventilator frequency. A regression model tested whether SHbT predicts cerebral pressure passivity and/or death/brain injury, controlling for birth gestational age and other factors.
Results
We studied 68 subjects with prematurity (n = 19), congenital heart disease (n = 11), and hypoxic–ischemic encephalopathy (n = 38). SHbT, sedative use, and pCO2 were positively associated, and circulating hemoglobin negatively associated, with cerebral pressure passivity (p < 0.001), which was positively associated with brain injury (p < 0.001).
Conclusion
In sick newborns, ventilator-related CBV fluctuations may predispose to cerebral pressure passivity, which may predispose to an adverse neonatal outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41.
Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–7.
Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–73.
Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010;41:1957–62.
Heldt T, Kashif FM, Sulemanji M, O’Leary HM, du Plessis AJ, Verghese GC. Continuous quantitative monitoring of cerebral oxygen metabolism in neonates by ventilator-gated analysis of NIRS recordings. Acta Neurochir Suppl. 2012;114:177–80.
Naulaers G, Meyns B, Miserez M, Leunens V, Van Huffel S, Casaer P, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology. 2007;92:120–6.
Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol. 2015;114:818–24.
Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44:591–5.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Govindan RB, Massaro A, Chang T, Vezina G, du Plessis A. A novel technique for quantitative bedside monitoring of neurovascular coupling. J Neurosci Methods. 2016;259:135–42.
Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–32.
Govindan RB, Massaro AN, Andescavage NN, Chang T, du Plessis A. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front Hum Neurosci. 2013;8:266.
O’Leary H, Gregas MC, Limperopoulos C, Zaretskaya I, Bassan H, Soul JS, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124:302–9.
Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24.
du Plessis AJ. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J Child Neurol. 2009;24:1127–40.
du Plessis AJ. Neurologic complications of cardiac disease in the newborn. Clin Perinatol. 1997;24:807–26.
Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: insights and future directions. Neuroimage. 2019;185:776–82.
Vesoulis ZA, Mathur AM. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front Pediatr. 2017;5:64.
Lou HC. The “lost autoregulation hypothesis” and brain lesions in the newborn—an update. Brain Dev. 1988;10:143–6.
Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990;117:119–25.
Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008;39:2531–7.
Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–25.
Lee JK, Yang ZJ, Wang B, Larson AC, Jamrogowicz JL, Kulikowicz E, et al. Noninvasive autoregulation monitoring in a swine model of pediatric cardiac arrest. Anesth Analg. 2012;114:825–36.
Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74:525–35.
Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40:1820–6.
Vesoulis ZA, Liao SM, Trivedi SB, Ters NE, Mathur AM. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr Res. 2016;79:453–9.
Perry BG, Lucas SJ, Thomas KN, Cochrane DJ, Mundel T. The effect of hypercapnia on static cerebral autoregulation. Physiol Rep. 2014;2:e12059.
Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas. 1999;20:265–75.
Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. 2016;20:128.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Funding
This study was supported by (1) internal special-purpose funds in the Fetal Medicine Institute at Children’s National and (2) the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01) and the National Institutes of Health Intellectual and Developmental Disabilities Research Consortium (U54 HD090257).
Author information
Authors and Affiliations
Contributions
VG performed data collection, performed data analysis, and drafted the initial paper. RG and TA-S performed data analysis. ANM and NNA aided in recruitment of subjects and worked to shape the design of the study. GV and JM performed radiological classification of MRI. YW performed statistical analysis and summarized the results. MM, CC, CS, and DR performed data collection. AP and RG conceptualized and designed the study, supervised data analysis. All authors participated in the interpretation of the results, reviewed, and endorsed the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Govindan, V., Govindan, R., Massaro, A.N. et al. Cerebral venous volume changes and pressure autoregulation in critically ill infants. J Perinatol 40, 806–811 (2020). https://doi.org/10.1038/s41372-020-0626-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-020-0626-0
This article is cited by
-
Towards quantitative assessment of cerebrovascular autoregulation in human neonates using ultrafast ultrasound imaging
Scientific Reports (2025)
-
Effect of the change of mechanical ventilation mode on cerebral oxygen saturation level in neonates
BMC Pediatrics (2023)
-
Cerebral venous volume changes and pressure autoregulation in critically ill infants: an editorial comment
Journal of Perinatology (2020)