Abstract
Objective
To characterize the prevalence of exchange transfusion (ET), clinical characteristics of infants receiving ET, and ET-associated morbidity and mortality.
Study design
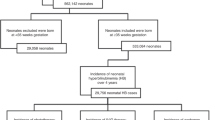
We conducted a multicenter cohort study of infants ≥23 weeks of gestational age (GA) with hyperbilirubinemia who underwent ET within 30 days of birth from 1997 to 2016. We examined clinical characteristics and adverse events after ET. We used multivariable logistic regression to examine the association between clinical risk factors and death.
Result
A total of 1252 infants were included; 4% died within 7 days of ET and 6% died before discharge. Compared with infants ≥37 weeks of GA, infants ≤29 weeks of GA had greater odds of death (adjusted odds ratio [95% confidence interval] = 20.08 [7.32, 55.07]).
Conclusions
Infants ≤ 29 weeks of GA had greater odds of death following ET compared with term infants. These data will support clinicians in evaluating risks and prognosis for infants who require ET.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–82. e1
Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175:587–90.
Truman P. Jaundice in the preterm infant. Paediatr Nurs. 2006;18:20–2.
Bhutani VK, Johnson L. Kernicterus in the 21st century: frequently asked questions. J Perinatol. 2009;29(Suppl 1):S20–4.
Bhutani VK, Johnson L. Synopsis report from the pilot USA Kernicterus Registry. J Perinatol. 2009;29(Suppl 1):S4–7.
Watchko JF, Claassen D. Kernicterus in premature infants: current prevalence and relationship to NICHD Phototherapy Study exchange criteria. Pediatrics. 1994;93:996–9.
Watchko JF, Maisels MJ. Jaundice in low birthweight infants: pathobiology and outcome. Arch Dis Child Fetal Neonatal Ed. 2003;88:F455–8.
Okumura A, Kidokoro H, Shoji H, Nakazawa T, Mimaki M, Fujii K, et al. Kernicterus in preterm infants. Pediatrics. 2009;123:e1052–8.
Brown AK, Kim MH, Wu PY, Bryla DA. Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics. 1985;75:393–400.
Nasseri F, Mamouri GA, Babaei H. Intravenous immunoglobulin in ABO and Rh hemolytic diseases of newborn. Saudi Med J. 2006;27:1827–30.
Newman TB, Maisels MJ. Evaluation and treatment of jaundice in the term newborn: a kinder, gentler approach. Pediatrics. 1992;89:809–18.
No authors listed. Practice parameter: management of hyperbilirubinemia in the healthy term newborn. American Academy of Pediatrics. Provisional Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia. Pediatrics. 1994;94:558–65.
Ives NK. Management of neonatal jaundice. Paediatr Child Health. 2011;21:270–6.
Chitty HE, Ziegler N, Savoia H, Doyle LW, Fox LM. Neonatal exchange transfusions in the 21st century: a single hospital study. J Paediatr Child Heal. 2013;49:825–32.
Flaherman VJ, Kuzniewicz MW, Escobar GJ, Newman TB. Total serum bilirubin exceeding exchange transfusion thresholds in the setting of universal screening. J Pediatr. 2012;160:796–800. e1
Hakan N, Zenciroglu A, Aydin M, Okumus N, Dursun A, Dilli D. Exchange transfusion for neonatal hyperbilirubinemia: an 8-year single center experience at a tertiary neonatal intensive care unit in Turkey. J Matern Neonatal Med. 2015;28:1537–41.
Steiner LA, Bizzarro MJ, Ehrenkranz RA, Gallagher PG. A decline in the frequency of neonatal exchange transfusions and its effect on exchange-related morbidity and mortality. Pediatrics. 2007;120:27–32.
Patra K, Storfer-Isser A, Siner B, Moore J, Hack M. Adverse events associated with neonatal exchange transfusion in the 1990s. J Pediatr. 2004;14:626–31.
Sabzehei MK, Basiri B, Shokouhi M, Torabian S. Complications of exchange transfusion in hospitalized neonates in two neonatal centers in Hamadan, a five-year experience. J Compr Pediatr. 2015;6:e20587.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps® Data Warehouse and the Pediatrix QualitySteps improvement project system-tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Olusanya BO, Iskander IF, Slusher TM, Wennberg RP. A decision-making tool for exchange transfusions in infants with severe hyperbilirubinemia in resource-limited settings. J Perinatol. 2016;36:338–41.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24.
Gunnink SF, Vlug R, Fijnvandraat K, Van Der Bom JG, Stanworth SJ, Lopriore E. Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol. 2014;7:387–95.
Roberts I. Neonatal thrombocytopenia: causes and management. Arch Dis Child Fetal Neonatal Ed. 2003;88:F359–64.
Jain A, Agarwal R, Sankar MJ, Deorari A, Paul VK. Hypocalcemia in the newborn. Indian J Pediatr. 2010;77:1123–8.
Vemgal P, Ohlsson A. Interventions for non-oliguric hyperkalaemia in preterm neonates. Cochrane Database Syst Rev. 2012;CD005257.
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8.
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88:S69–74.
Ramani M, Ambalavanan N. Feeding practices and necrotizing enterocolitis. Clin Perinatol. 2013;40:1–10.
Zwiers C, Scheffer-Rath ME, Lopriore E, de Haas M, Liley HG. Immunoglobulin for alloimmune hemolytic disease in neonates. Cochrane Database Syst Rev. 2018;3:CD003313.
Yu C, Li H, Zhang Q, He H, Chen X, Hua Z. Report about term infants with severe hyperbilirubinemia undergoing exchange transfusion in Southwestern China during an 11-year period, from 2001 to 2011. PLoS ONE. 2017;12:e0179550.
Jackson JC. Adverse events associated with exchange transfusion in healthy and ill newborns. Pediatrics. 1997;99:E7.
Figueras-Aloy J, Rodriguez-Miguelez JM, Iriondo-Sanz M, Salvia-Roiges MD, Botet-Mussons F, Carbonell-Estrany X. Intravenous immunoglobulin and necrotizing enterocolitis in newborns with hemolytic disease. Pediatrics. 2010;125:139–44.
Platt MJ. Outcomes in preterm infants. Public Health. 2014;128:399–403.
Boghossian NS, Geraci M, Edwards EM, Horbar JD. Morbidity and mortality in small for gestational age infants at 22 to 29 weeks’ gestation. Pediatrics. 2018;141. pii: e20172533. https://doi.org/10.1542/peds.2017-2533.
Acknowledgements
PTN Steering Committee Members: Daniel K. Benjamin Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Scottsdale, AZ; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee. The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor. PTN Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL
Funding
This work was funded under the National Institute of Child Health and Human Development (NICHD) contract (HHSN275201000003I) for the Pediatric Trials Network (PI Danny Benjamin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program (grant #5R25HD076475-07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RGG has received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/). The authors have no other conflicts of interest relevant to this article to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wolf, M.F., Childers, J., Gray, K.D. et al. Exchange transfusion safety and outcomes in neonatal hyperbilirubinemia. J Perinatol 40, 1506–1512 (2020). https://doi.org/10.1038/s41372-020-0642-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-020-0642-0
This article is cited by
-
Hemolytic disease of the fetus and newborn: rapid review of postnatal care and outcomes
BMC Pregnancy and Childbirth (2023)
-
Intravenous immunoglobulin G therapy for neonatal hyperbilirubinemia
Pediatric Research (2023)
-
Role of ursodeoxycholic acid in neonatal indirect hyperbilirubinemia: a systematic review and meta-analysis of randomized controlled trials
Italian Journal of Pediatrics (2022)
-
Understanding the risk factors for adverse events during exchange transfusion in neonatal hyperbilirubinemia using explainable artificial intelligence
BMC Pediatrics (2022)
-
History and current standard of postnatal management in hemolytic disease of the fetus and newborn
European Journal of Pediatrics (2022)