Abstract
Objective
Characterize the use, efficacy, and safety of poractant alfa and calfactant surfactants compared to beractant in preterm infants receiving late surfactant.
Study design
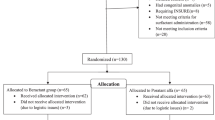
We included infants <37 weeks gestational age (GA) discharged from Pediatrix Medical Group-managed neonatal intensive care units (1997–2017). Efficacy and safety outcomes of interest were analyzed.
Results
Of 184,770 infants administered surfactant at any time, 7846 (4.23%) received late surfactant at a median (25th, 75th percentile) PNA of 8 days (3, 22); specifically, 2976 received poractant alfa (38%), 2890 beractant (37%), and 1936 calfactant (25%). We identified no significant differences in composite efficacy or safety outcomes between surfactants in the primary analysis, but 33–36 week GA infants administered poractant alfa had significantly greater odds of developing a safety event.
Conclusions
Compared to beractant, there is no evidence of overall superior efficacy or safety of poractant alfa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gallacher DJ, Hart K, Kotecha S. Common respiratory conditions of the newborn. Breathe. 2016;12:30–42.
Halliday HL. The fascinating story of surfactant. J Paediatr Child Health. 2017;53:327–32.
Taylor G, Jackson W, Hornik CP, Koss A, Mantena S, Homsley K, et al. Surfactant administration in preterm infants: drug development opportunities. J Pediatr. 2019;208:163–8.
Jeon GW. Surfactant preparations for preterm infants with respiratory distress syndrome: past, present, and future. Korean J Pediatr. 2019;62:155–61.
Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63.
Fujii AM, Patel SM, Allen R, Doros G, Guo CY, Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol. 2010;30:665–70.
Gharehbaghi MM, Sakha SH, Ghojazadeh M, Firoozi F. Complications among premature neonates treated with beractant and poractant alfa. Indian J Pediatr. 2010;77:751–4.
Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol. 2009;29:S38–43.
Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21:109–19.
Chiesi USA, Inc. Curosurf (poractant alfa suspension) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020744s028lbl.pdf. 2014. Accessed 30 June 2020.
ONY Biotech Inc. Infasurf—calfactant suspension [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020521s024lbl.pdf. 2011. Accessed 30 June 2020.
AbbVie Inc. Survanta—beractant suspension [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020032s045lbl.pdf. 2019. Accessed 30 June 2020.
Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, Eichenwald EC, et al. Randomized trial of late surfactant treatment in ventilated preterm infants receiving inhaled nitric oxide. J Pediatr. 2016;168:23–9.e4.
Keller RL, Eichenwald EC, Hibbs AM, Rogers EE, Wai KC, Black DM, et al. The randomized, controlled trial of late surfactant: effects on respiratory outcomes at 1-year corrected age. J Pediatr. 2017;183:19–25.e2.
Keller RL, Merrill JD, Black DM, Steinhorn RH, Eichenwald EC, Durand DJ, et al. Late administration of surfactant replacement therapy increases surfactant protein-B content: a randomized pilot study. Pediatr Res. 2012;72:613–9.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system-tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1.
Greenberg JM, Poindexter BB, Shaw PA, Bellamy SL, Keller RL, Moore PE, et al. Respiratory medication use in extremely premature (<29 weeks) infants during initial NICU hospitalization: Results from the prematurity and respiratory outcomes program. Pediatr Pulmonol. 2020;55:360–8.
Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108.
Attar MA, Becker MA, Dechert RE, Donn SM. Immediate changes in lung compliance following natural surfactant administration in premature infants with respiratory distress syndrome: a controlled trial. J Perinatol. 2004;24:626–30.
Bloom BT, Clark RH. Comparison of Infasurf (calfactant) and Survanta (beractant) in the prevention and treatment of respiratory distress syndrome. Pediatrics. 2005;116:392–9.
Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics. 1997;100:31–8.
Logan JW, Moya FR. Animal-derived surfactants for the treatment and prevention of neonatal respiratory distress syndrome: summary of clinical trials. Ther Clin Risk Manag. 2009;5:251–60.
Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, Rojas-Reyes MX. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2015;12:CD010249.
Tridente A, De Martino L, De, Luca D. Porcine vs bovine surfactant therapy for preterm neonates with RDS: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes. Respir Res. 2019;20:28.
Hascoët J-M, Picaud J-C, Ligi I, Blanc T, Moreau F, Pinturier M-F, et al. Late surfactant administration in very preterm neonates with prolonged respiratory distress and pulmonary outcome at 1 year of age: a randomized clinical trial. JAMA Pediatr. 2016;170:365–72.
Paul S, Rao S, Kohan R, McMichael J, French N, Zhang G, et al. Poractant alfa versus beractant for respiratory distress syndrome in preterm infants: a retrospective cohort study. J Paediatr Child Health. 2013;49:839–44.
Bos AP, Tibboel D, Hazebroek FW, Molenaar JC, Lachmann B, Gommers D. Surfactant replacement therapy in high-risk congenital diaphragmatic hernia. Lancet. 1991;338:1279.
Deshpande S, Suryawanshi P, Ahya K, Maheshwari R, Gupta S. Surfactant therapy for early onset pneumonia in late preterm and term neonates needing mechanical ventilation. J Clin Diagn Res. 2017;11:SC09–12.
El Shahed AI, Dargaville PA, Ohlsson A, Soll R. Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev. 2014;12:CD002054.
Merrill JD, Ballard RA, Cnaan A, Hibbs AM, Godinez RI, Godinez MH, et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr Res. 2004;56:918–26.
Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. 2009;123:89–96.
Funding
Supported by DCRI’s R25 STAR Program (grant #5R25HD076475-07).
Author information
Authors and Affiliations
Contributions
Drafting, revising, and final paper preparation: MDL, CPH, and KOZ. Oversight, funding: KOZ. Drafting, review: SK, OU, SED, RMT, AL, SB, and RHC. Initial concept, revising: WMJ, ML, CPH, and KOZ. Statistical analysis: CPH, RGG. Provided data: RHC.
Corresponding author
Ethics declarations
Competing interests
MDL: research support from Duke BIGGER Program. SB: support from the National Institutes of Health, FDA, PCORI, the Rheumatology Research Foundation’s Scientist Development Award, the Thrasher Research Fund, the Childhood Arthritis and Rheumatology Research Alliance, and consulting for UCB. KOZ: support from the National Institutes of Health (National Institute of Child Health and Human Development (K23 HD091398, HHSN275201000003I), the US FDA (UG3/UH3 FD 006797), the Duke Clinical and Translational Science Award (KL2TR001115-03), and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). All other authors report no competing interests relevant to this paper.
Ethical approval
The Duke IRB approved this study with a waiver of consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SK, OU, SED, RMT, AL: High school or college student affiliated with the Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program.
Supplementary information
Rights and permissions
About this article
Cite this article
Lane, M.D., Kishnani, S., Udemadu, O. et al. Comparative efficacy and safety of late surfactant preparations: a retrospective study. J Perinatol 41, 2639–2644 (2021). https://doi.org/10.1038/s41372-021-01142-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-021-01142-2
This article is cited by
-
Surfactant treatment at birth in a contemporary cohort of preterm infants with bronchopulmonary dysplasia
Journal of Perinatology (2024)
-
Use of surfactant beyond respiratory distress syndrome, what is the evidence?
Journal of Perinatology (2024)