Abstract
Objective
Determine the accuracy of diagnostic codes in identifying Prenatal Opioid Exposure (POE) and Neonatal Opioid Withdrawal Syndrome (NOWS).
Study design
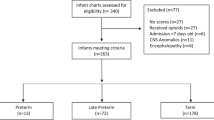
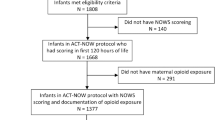
A cross-sectional study of 374,222 mother-infant dyads with delivery from 01/01/2010 to 12/31/2019. We ascertained maternal diagnostic codes for opioid use during pregnancy and infant diagnostic codes for drug exposure and withdrawal. We assessed sensitivity and positive predictive value (PPV) for POE and NOWS, defined using laboratory, pharmacy, and clinical data.
Results
Maternal codes had low sensitivity (36.4%) and PPV (34.7%) for POE. Infant codes for drug exposure were neither sensitive for POE (14%) nor NOWS (31.6%) and had low PPV. Codes for newborn withdrawal had low sensitivity (31.6%) for detecting NOWS, but high PPV (85%). Sensitivity improved (95.1%) for NOWS requiring pharmacologic treatment.
Conclusions
Diagnostic codes identify POE and NOWS poorly. Improved case identification would include pharmacy and laboratory results, and clearly defined criteria for evidence of withdrawal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Code availability
All analyses were performed using SAS version 9.4. The code used is available upon request.
Data availability
The datasets generated for this study are stored at the KPNC Division of Research. Deidentified data can be provided upon reasonable request to the corresponding author, and with permission from the KPNC Institutional Review Board.
References
Summers AD, Ailes EC, Bohm MK, Tran EL, Broussard CS, Frey MT, et al. Opioid prescription claims among women aged 15-44 years-United States, 2013–2017. J Opioid Manag. 2021;17:125–33.
Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA. 2021;325:146–55.
Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics. 2019;143:3.
Prevention CfDCa. Public health grand rounds: surveillance for emerging threats to pregnant women and infants: data for action Atlanta, GA2018 [Available from: https://www.cdc.gov/grand-rounds/pp/2018/20180918-pregnancy-threats.html.
Chiang KV, Okoroh EM, Kasehagen LJ, Garcia-Saavedra LF, Ko JY. Standardization of state definitions for neonatal abstinence syndrome surveillance and the opioid crisis. Am J Public Health. 2019;109:1193–7.
Jilani SM, Frey MT, Pepin D, Jewell T, Jordan M, Miller AM, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:6–10.
Phillips-Bell GS, Holicky A, Lind JN, Sappenfield WM, Hudak ML, Petersen E, et al. Assessing the burden of neonatal abstinence syndrome: validation of ICD-9-CM data, Florida, 2010–2011. J Public Health Manag Pr. 2020;26:E1–E8.
Elmore AL, Tanner JP, Lowry J, Lake-Burger H, Kirby RS, Hudak ML, et al. Diagnosis codes and case definitions for neonatal abstinence syndrome. Pediatrics. 2020;146:6.
Epidemiologists CoSaT. Council of State and Territorial Epidemiologists Neonatal Abstinence Syndrome Standardized Case Definition Atlanta, GA2019 [Available from: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2019ps/final/19-MCH-01_final_7.31.19.pdf.
Gordon N. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: Statistics from the 2011-12 California Health Interview Survey. 2015.
Chimmula S, Dhuru R, Folck B, Gul J, Lee M, Ng D, et al. PS2-44: VDW data sources: Kaiser Permanente Northern California. Clin Med Res. 2012;10:193.
Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser permanente medical care program. In: Strom BL, editor. Pharmacoepidemiology. 4 ed. New York: Wiley; 2005. p. 241–59.
Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2:141–58.
Jones HE, Harrow C, O’Grady KE, Crocetti M, Jansson LM, Kaltenbach K. Neonatal abstinence scores in opioid-exposed and nonexposed neonates: a blinded comparison. J Opioid Manag. 2010;6:409–13.
Jilani SM, Jones HE, Davis JM. Implementation of a standardized clinical definition of opioid withdrawal in the neonate: challenges and opportunities. JAMA. 2022;327:1643–4.
Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124:1031–9.
MacVicar S, Kelly LE. Systematic mixed-study review of nonpharmacological management of neonatal abstinence syndrome. Birth. 2019;46:428–38.
Grossman MR, Lipshaw MJ, Osborn RR, Berkwitt AK. A novel approach to assessing infants with neonatal abstinence syndrome. Hosp Pediatr. 2018;8:1–6.
Grossman MR, Berkwitt AK, Osborn RR, Xu Y, Esserman DA, Shapiro ED, et al. An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics. 2017;139:e1–e8.
Camden A, Ray JG, To T, Gomes T, Bai L, Guttmann A. Identification of prenatal opioid exposure within health administrative databases. Pediatrics. 2021;147:1–9.
Doherty KM, Scott TA, Morad A, Crook T, McNeer E, Lovell KS, et al. Evaluating definitions for neonatal abstinence syndrome. Pediatrics. 2021;147:1–6.
Goyal S, Saunders KC, Moore CS, Fillo KT, Ko JY, Manning SE, et al. Identification of substance-exposed newborns and neonatal abstinence syndrome using ICD-10-CM - 15 hospitals, Massachusetts, 2017. MMWR Morb Mortal Wkly Rep. 2020;69:951–5.
Maalouf FI, Cooper WO, Stratton SM, Dudley JA, Ko J, Banerji A, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143:1–6.
Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135:842–50.
Sujan AC, Quinn PD, Rickert ME, Wiggs KK, Lichtenstein P, Larsson H, et al. Maternal prescribed opioid analgesic use during pregnancy and associations with adverse birth outcomes: A population-based study. PLoS Med. 2019;16:e1002980.
Brogly SB, Velez MP, Werler MM, Li W, Camden A, Guttmann A. Prenatal opioid analgesics and the risk of adverse birth outcomes. Epidemiology. 2021;32:448–56.
Falk J, Dahl M, Raymond CB, Chateau D, Katz A, Leong C, et al. Opioid use during pregnancy: a population-based cohort study. CMAJ Open. 2017;5:E517–23.
D’Apolito KC. Assessing neonates for neonatal abstinence: are you reliable? J Perinat Neonatal Nurs. 2014;28:220–31.
Hudak ML, Tan RC, Committee On D, Committee On F, Newborn, American Academy of P. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–60.
Jones HE, Seashore C, Johnson E, Horton E, O’Grady KE, Andringa K, et al. Psychometric assessment of the neonatal abstinence scoring system and the MOTHER NAS scale. Am J Addict. 2016;25:370–3.
Funding
This project was supported by FDA Contract 75F40119C10101. CIC receives funds through her institution from the Opioid Postmarketing Requirements (PMR) Consortium for research on risks of long-term prescription opioid use, conducted as part of a Food and Drug Administration postmarketing requirement for extended-release and long-acting opioid analgesics. The remaining authors have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Contributions
MWK conceptualized and designed the study, obtained funding, acquired the data, carried out all data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. CIC, SDC, LAC, SDP, and MH assisted in interpreting the analyses, assisted in writing the initial draft, and reviewed and revised the manuscript. SL was responsible for acquiring the data, building the datasets, and reviewing and revising the manuscript. EMW reviewed the data, performed chart review for data accuracy when needed, and reviewed and revised the manuscript. LSS assisted in the conceptualization and design of the study, obtained funding, reviewed data analyses, assisted in writing the initial draft and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed and approved by the following boards. Individual consent of subjects was waived. Kaiser Permanente Northern California Institutional Review Board (#1507334). California Committee for the Protection of Human Subjects (#2021-043). The study is in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuzniewicz, M.W., Campbell, C.I., Li, S. et al. Accuracy of diagnostic codes for prenatal opioid exposure and neonatal opioid withdrawal syndrome. J Perinatol 43, 293–299 (2023). https://doi.org/10.1038/s41372-022-01518-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-022-01518-y