Abstract
Objective
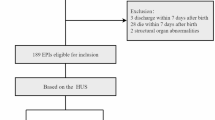
Intraventricular hemorrhage (IVH) is a common cause of brain injury in preterm infants. Fresh human milk (HM) contains stem cells (SCs) that could potentially be delivered via intranasal HM (IHM). In this IHM pilot study, we describe outcomes.
Study design
Infants <33 weeks gestation with IVH were given IHM until maximum 28 days of age. Short-term neurologic outcomes and follow-up testing were compared to historic HM-fed infants. Longitudinal outcomes were plotted using linear mixed models. Weighted G-computation quantified treatment effects. Propensity score models calculated inverse probability weights for IVH grade, gestational age, and sex.
Result
37 infants (35.1% grade 3-4 IVH) were compared to 191 historic controls (17.8% grade 3-4 IVH). Post-hemorrhagic ventricular dilatation was common (25.7% IHM patients). Most weighted outcomes, although not significant, favored IHM at 4-12 and 18 months corrected age.
Conclusion
This phase 1 study suggests powered trials of IHM for brain injury are needed.
Clinical Trial Registry Name
clinicaltrials.gov identifier NCT04225286
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Relevant data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Mukerji A, Shah V, Shah PS. Periventricular/Intraventricular Hemorrhage and Neurodevelopmental Outcomes: A Meta-analysis. Pediatrics. 2015;136:1132–43.
Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 2014;57:251–6.
Whitelaw A. Core Concepts: Intraventricular Hemorrhage. NeoReviews. 2011;12:e94–101.
Aydın MŞ, Yiğit EN, Vatandaşlar E, Erdoğan E, Öztürk G. Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Sci Rep. 2018;8:14289.
Mitsialis SA, Kourembanas S. Stem Cell-Based Therapies for the Newborn Lung and Brain: Possibilities and Challenges. Semin Perinatol. 2016;40:138–51.
Parodi A, Govaert P, Horsch S, Bravo MC, Ramenghi LA. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr Res. 2020;87:13–24.
Briere CE, McGrath JM, Jensen T, Matson A, Finck C. Breast Milk Stem Cells: Current Science and Implications for Preterm Infants. Adv Neonatal Care. 2016;16:410–9.
Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164:973–979.e1.
Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal Stem Cells: The Magic Cure for Intraventricular Hemorrhage? Cell Transpl. 2017;26:439–48.
Ninomiya K, Iwatsuki K, Ohnishi Yichiro, Ohkawa T, Yoshimine T. Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J Neurosurg Spine. 2015;23:111–9.
Mukai T, Mori Y, Shimazu T, Takahashi A, Tsunoda H, Yamaguchi S, et al. Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience. 2017;355:175–87.
Hosseini SM, Talaei-khozani T, Sani M, Owrangi B. Differentiation of Human Breast-Milk Stem Cells to Neural Stem Cells and Neurons. Neurol Res Int. 2014;2014:807896.
Keller T, Körber F, Oberthuer A, Schafmeyer L, Mehler K, Kuhr K, et al. Intranasal breast milk for premature infants with severe intraventricular hemorrhage-an observation. Eur J Pediatr. 2019;178:199–206.
Keller T, Wengenroth L, Smorra D, Probst K, Kurian L, Kribs A, et al. Novel DRAQ5TM/SYTOX® Blue Based Flow Cytometric Strategy to Identify and Characterize Stem Cells in Human Breast Milk. Cytom B Clin Cytom. 2019;96:480–9.
Borhani-Haghighi M, Navid S, Mohamadi Y. The Therapeutic Potential of Conditioned Medium from Human Breast Milk Stem Cells in Treating Spinal Cord Injury. Asian Spine J. 2020;14:131–8.
Gong Z, Wang C, Ni L, Ying L, Shu J, Wang J, et al. An injectable recombinant human milk fat globule-epidermal growth factor 8-loaded copolymer system for spinal cord injury reduces inflammation through NF-κB and neuronal cell death. Cytotherapy. 2020;22:193–203.
de Weerth C, Aatsinki AK, Azad MB, Bartol FF, Bode L, Collado MC, et al. Human milk: From complex tailored nutrition to bioactive impact on child cognition and behavior. Crit Rev Food Sci Nutr. 2023;63:7945–82.
Hoban R, Gallipoli A, Signorile M, Mander P, Gauthier-Fisher A, Librach C, et al. Feasibility of intranasal human milk as stem cell therapy in preterm infants with intraventricular hemorrhage. J Perinatol. 2024. https://doi.org/10.1038/s41372-024-01982-8.
Nabetani M, Shintaku H, Hamazaki T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2018;83:356–63.
Twigger AJ, Hodgetts S, Filgueira L, Hartmann PE, Hassiotou F. From breast milk to brains: the potential of stem cells in human milk. J Hum Lact. 2013;29:136–9.
Eliks M, Gajewska E. The Alberta Infant Motor Scale: A tool for the assessment of motor aspects of neurodevelopment in infancy and early childhood. Front Neurol. 2022;13:927502.
Case-Smith J. Reliability and Validity of the Posture and Fine Motor Assessment of Infants. Occup Ther J Res. 1989;9:259–72.
Squires J, Bricker D Ages & Stages Questionnaires®, Third Edition (ASQ-3™). APA PsycTests; 2020. https://doi.org/10.1037/t11523-000.
Bayley N Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III®). APA PsycTests; 2005. https://doi.org/10.1037/t14978-000.
Robins DL, Barton M, Fein D Modified Checklist for Autism in Toddlers, Revised with Follow-up (M-CHAT-R/F). APA PsycTests; 2014. https://doi.org/10.1037/t67574-000.
Ren J, Cislo P, Cappelleri JC, Hlavacek P, DiBonaventura M. Comparing g-computation, propensity score-based weighting, and targeted maximum likelihood estimation for analyzing externally controlled trials with both measured and unmeasured confounders: a simulation study. BMC Med Res Methodol. 2023;23:18.
Loiseau N, Trichelair P, He M, Andreux M, Zaslavskiy M, Wainrib G, et al. External control arm analysis: an evaluation of propensity score approaches, G-computation, and doubly debiased machine learning. BMC Med Res Methodol. 2022;22:335.
Adams-Chapman I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin Perinatol. 2018;45:453–66.
DeMauro SB. Neurodevelopmental outcomes of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2021;56:3509–17.
Belfort MB, Inder TE. Human Milk and Preterm Infant Brain Development: A Narrative Review. Clin Ther. 2022;44:612–21.
Witkowska-Zimny M, Majczyna D. How Knowledge about Stem Cells Influences Attitudes towards Breastfeeding: Case Study of Polish Women. Int J Environ Res Public Health. 2021;18:2382.
Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74.
Acknowledgements
We are grateful to the families who participated, as well as the bedside nursing, medical, and respiratory staff who made this study possible during the pandemic.
Funding
Funding for this project was obtained through The New Frontiers in Research Fund – Exploration Grant 2018, Canadian Tri-Agencies (grant ID NFRFE-2018-01610).
Author information
Authors and Affiliations
Contributions
RH was responsible for conceptualization, funding acquisition, methodology, project administration, supervision, investigation, data collection, and writing (both original draft and review/editing). AG, a trainee at the time of the study, was responsible for data collection, data visualization, and writing (both original draft and review/editing). SU was responsible for funding acquisition, supervision, investigation, data collection, and writing (review/editing). AES and DW were responsible for funding acquisition, investigation, data collection, and writing (review/editing). SF and MS provided computing resources and software, statistical methodology and formal analysis, and writing (both original draft and review/editing).
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest relevant to this article to disclose. RH is currently on the clinical advisory board for Medela America (was not on the board during this study.)
Consent to participate statement
Written informed consent was obtained from the guardian of eligible participants prior to participation in the intervention portion of the study.
Ethics approval
This study protocol was reviewed and approved by Clinical Trials Ontario, project ID 1911.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gallipoli, A., Unger, S., El Shahed, A. et al. Outcomes after intranasal human milk therapy in preterm infants with intraventricular hemorrhage. J Perinatol 45, 202–207 (2025). https://doi.org/10.1038/s41372-024-02147-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-024-02147-3