Abstract
Objective
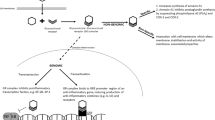
Short-term treatment efficacy of systemic dexamethasone (DEX) in preterm infants with bronchopulmonary dysplasia (BPD) is highly variable. Our objective was to assess if salivary cortisol may serve as a reliable biomarker of steroid response.
Study design
Multi-site prospective observational cohort study. Salivary cortisol was measured before and after DEX treatment. Respiratory Severity Score (RSS) quantified clinical response.
Results
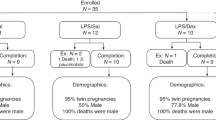
Fifty-four infants with median (inter-quartile range) gestational age of 25.1 (24.1,26.5) weeks initiated DEX at 30 (23,48) days’ postnatal age. Median baseline and post-treatment cortisol levels were 0.3 (0.2,0.6) μg/dl; 8.3 (5.5,16.5) nmol/L and 0.2 (0.1,0.3) μg/dl; 5.5 (2.8,8.3) nmol/L, respectively. RSS values decreased by a median of 3.1(1.6,5.0) Change in RSS did not correlate with baseline cortisol or change in cortisol levels.
Conclusion
In this first study to assess salivary cortisol as a biomarker for DEX response in BPD, salivary cortisol did not predict dexamethasone response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data reported in this manuscript can be requested through contacting the corresponding author. De-identified or aggregate portions of the data may be shared within the bounds of the original informed consent process.
References
Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011;18:265–70.
Hochwald O, Bentur L, Haddad Y, Hanna M, Zucker-Toledano M, Mainzer G, et al. Cardiopulmonary Exercise Testing in Childhood in Late Preterms: Comparison to Early Preterms and Term-Born Controls. J Pers Med. 2022;12:1547.
Harmon HM, Jensen EA, Tan S, Chaudhary AS, Slaughter JL, Bell EF, et al. Timing of postnatal steroids for bronchopulmonary dysplasia: association with pulmonary and neurodevelopmental outcomes. J Perinatol : Off J Calif Perinat Assoc. 2020;40:616–27.
Cuna A, Lewis T, Dai H, Nyp M, Truog WE. Timing of postnatal corticosteroid treatment for bronchopulmonary dysplasia and its effect on outcomes. Pediatr Pulmonol. 2019;54:165–70.
Cuna A, Lagatta JM, Savani RC, Vyas-Read S, Engle WA, Rose RS, et al. Association of time of first corticosteroid treatment with bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. 2021;56:3283–92.
Lewis T, Truog W, Norberg M, Ballard PL, Torgerson D, Group TS. Genetic variation in CRHR1 is associated with short-term respiratory response to corticosteroids in preterm infants at risk for bronchopulmonary dysplasia. Pediatr Res. 2019;85:625–33.
Cuna A, Govindarajan S, Oschman A, Dai H, Brophy K, Norberg M, et al. A comparison of 7-day versus 10-day course of low-dose dexamethasone for chronically ventilated preterm infants. J Perinatol. 2017;37:301–5.
Cuna A, Lewis T, Oschman A, Dai HY, Brophy K, Norberg M, et al. Outcomes of Infants Who Failed to Extubate despite Systemic Corticosteroids. Am J Perinatol. 2017;34:1458–63.
Rocha G, Calejo R, Arnet V, de Lima FF, Cassiano G, Diogo I, et al. The use of two or more courses of low-dose systemic dexamethasone to extubate ventilator-dependent preterm neonates may be associated with a higher prevalence of cerebral palsy at two years of corrected age. Early Hum Dev. 2024;194:106050.
Cummings JJ, Pramanik AK, Committee on fetus and newborn. Postnatal Corticosteroids to Prevent or Treat Chronic Lung Disease Following Preterm Birth. Pediatrics. 2022;149:e2022057530.
Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics 2005;115:655–61.
Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatrics. 2014;165:1258–60.
Jensen EA, Wiener LE, Rysavy MA, Dysart KC, Gantz MG, Eichenwald EC, et al. Assessment of Corticosteroid Therapy and Death or Disability According to Pretreatment Risk of Death or Bronchopulmonary Dysplasia in Extremely Preterm Infants. JAMA Netw Open. 2023;6:e2312277.
Bamat NA, Kirpalani H, Feudtner C, Jensen EA, Laughon MM, Zhang H, et al. Medication use in infants with severe bronchopulmonary dysplasia admitted to United States children’s hospitals. J Perinatol Off J Calif Perinat Assoc. 2019;39:1291–9.
Nath S, Reynolds AM, Lakshminrusimha S, Ma C, Hudak ML, Ryan RM. Retrospective Analysis of Short-Term Respiratory Outcomes of Three Different Steroids Used in Clinical Practice in Intubated Preterm Infants. Am J Perinatol. 2020;37:1425–31.
Boscarino G, Cardilli V, Conti MG, Liguori F, Repole P, Parisi P, et al. Outcomes of postnatal systemic corticosteroids administration in ventilated preterm newborns: a systematic review of randomized controlled trials. Front Pediatr. 2024;12:1344337.
Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–35.
Kielt MJ, Logan JW, Backes CH, Conroy S, Reber KM, Shepherd EG, et al. Noninvasive Respiratory Severity Indices Predict Adverse Outcomes in Bronchopulmonary Dysplasia. J Pediatrics. 2022;242:129–36.e2
Liew Z, Fenton AC, Harigopal S, Gopalakaje S, Brodlie M, O’Brien CJ. Physiological effects of high-flow nasal cannula therapy in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2020;105:87–93.
Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. xxi, 567 p. p.
Finken MJJ, van der Voorn B, Hollanders JJ, Ruys CA, de Waard M, van Goudoever JB, et al. Programming of the Hypothalamus-Pituitary-Adrenal Axis by Very Preterm Birth. Ann Nutr Metab. 2017;70:170–4.
Kamrath C, Hartmann MF, Boettcher C, Wudy SA. Reduced activity of 11beta-hydroxylase accounts for elevated 17alpha-hydroxyprogesterone in preterms. J Pediatrics. 2014;165:280–4.
Iacobelli S, Allamele-Moutama K, Lorrain S, Gouyon B, Gouyon JB, Bonsante F, et al. Postnatal corticosteroid exposure in very preterm infants: A French cohort study. Front Pharm. 2023;14:1170842.
Lewis T, Truog W, Nelin L, Napolitano N, McKinney RL. Pharmacoepidemiology of Drug Exposure in Intubated and Non-Intubated Preterm Infants With Severe Bronchopulmonary Dysplasia. Front Pharm. 2021;12:695270.
Hathout Y, Conklin LS, Seol H, Gordish-Dressman H, Brown KJ, Morgenroth LP, et al. Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children. Sci Rep. 2016;6:31727.
Zhu H, Tian Y, Cheng H, Zheng Y, Wang W, Bao T, et al. A clinical study on plasma biomarkers for deciding the use of adjuvant corticosteroid therapy in bronchopulmonary dysplasia of premature infants. Int J Med Sci. 2021;18:2581–8.
Bettendorf M, Albers N, Bauer J, Heinrich UE, Linderkamp O, Maser-Gluth C. Longitudinal evaluation of salivary cortisol levels in full-term and preterm neonates. Horm Res. 1998;50:303–8.
Kari MA, Raivio KO, Stenman UH, Voutilainen R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res. 1996;40:319–24.
Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE Jr. Correlation between plasma and salivary cortisol levels in preterm infants. J Pediatrics. 2002;140:116–8.
Funding
T Lewis, W Truog: NICHD R21 (1R21HD101111), The Sellers Chair in Pharmacology and Pharmacogenetics, SickKids Foundation
Author information
Authors and Affiliations
Contributions
TL and WT conceptualized and designed the study, designed the data collection instruments, analyzed the data, drafted the initial manuscript and reviewed the final manuscript. EJ, SC, JS, MK, NPI, CN contributed to study design, recruited patients for the study, supervised colletion of samples and data, and critically reviewed and contributed to revisions of the manuscript. CG was the primary research coordinator for the study, designed data collection tools, collected data and aided in data analysis. HY analyzed the data and contributed to initial manuscript drafting and final manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Children’s Mercy Hospital IRB approved the study at the primary site in Kansas City, Missouri (REB #1000080681). Each site had local IRB approval prior to patient enrollment and data collection. All methods were performed in accordance with the relevant regulations and IRB approved protocol. Informed consent was obtained from all parents of infants in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lewis, T., Jensen, E.A., Courtney, S. et al. Salivary cortisol is not associated with dexamethasone response in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol (2024). https://doi.org/10.1038/s41372-024-02177-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-02177-x