Abstract
Objective
Characterize changes in neonatal energy metabolism hormones in first postnatal week.
Study design
Concentrations of leptin, adiponectin, insulin, resistin, and ghrelin were measured in cord and infant blood collected in the first postnatal week in a prospective cohort of term and preterm infants. Change over time in each hormone was modeled using linear mixed effects regression.
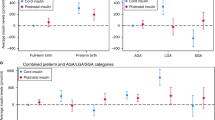
Result
Among 106 infants, 65 (61%) were preterm and 44 (42%) were exposed to diabetes (DM) in pregnancy. DM group had higher leptin [between group difference β 2.5 (95%CI: 1.72–3.70)] and resistin [β 1.5 (95%CI:1.14, 1.92)] and lower ghrelin [β 0.49] [95%CI: (0.32–0.76)] versus non-DM group. Preterm infants had lower adiponectin [β 0.80 (95%CI: 0.67–0.96)] versus term group. Insulin varied by DM and prematurity (interaction-term p value < 0.05).
Conclusion
In this cohort, hormone concentrations varied by DM and prematurity. Early alterations in energy metabolism hormones may reflect changes in developmental programming which persist in the early postnatal period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N. Engl J Med. 1983;308:242–5.
Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diab Care. 2017;40:679–86.
Lowe WL, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diab Care. 2019;42:372–80.
Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106:1409–37.
Buck CO, Shabanova V, Taylor SN. Growth patterns among late preterm infants of mothers with diabetes. J Matern Fetal Neonatal Med. 2022;35:10116–23.
Buck CO, Shabanova V, Clark RH, Taylor SN. Diabetes in pregnancy, neonatal morbidities, and early growth in moderate or late preterm infants. Pediatrics. 2023;152:e2023061285.
Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharm Sin. 2018;39:1176–88.
Teague AM, Fields DA, Aston CE, Short KR, Lyons TJ, Chernausek SD. Cord blood adipokines, neonatal anthropometrics and postnatal growth in offspring of Hispanic and Native American women with diabetes mellitus. Reprod Biol Endocrinol. 2015;13:68.
Shang M, Dong X, Hou L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J Obstet Gynaecol Res. 2018;44:637–46.
Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–60.
Ng PC, Lee CH, Lam CWK, Wong E, Chan IHS, Fok TF. Plasma ghrelin and resistin concentrations are suppressed in infants of insulin-dependent diabetic mothers. J Clin Endocrinol Metab. 2004;89:5563–8.
Buck CO, Eliot MN, Kelsey KT, Chen A, Kalkwarf H, Lanphear BP, et al. Neonatal adipocytokines and longitudinal patterns of childhood growth. Obes (Silver Spring). 2019;27:1323–30.
Tan K, Tint MT, Michael N, Yap F, Chong YS, Tan KH, et al. Determinants of cord blood adipokines and association with neonatal abdominal adipose tissue distribution. Int J Obes. 2022;46:637–45.
Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 1999;84:1145–8.
Arroyo-Jousse V, Jaramillo A, Castaño-Moreno E, Lépez M, Carrasco-Negüe K, Casanello P. Adipokines underlie the early origins of obesity and associated metabolic comorbidities in the offspring of women with pregestational obesity. Biochimica et Biophys Acta (BBA) - Mol Basis Dis. 2020;1866:165558.
Takaya J, Yamato F, Higashino H, Kaneko K. Intracellular magnesium and adipokines in umbilical cord plasma and infant birth size. Pediatr Res. 2007;62:700–3.
Simpson J, Smith ADAC, Fraser A, Sattar N, Lindsay RS, Ring SM, et al. Programming of Adiposity in Childhood and Adolescence: Associations With Birth Weight and Cord Blood Adipokines. J Clin Endocrinol Metab. 2017;102:499–506.
Wallace AM. Measurement of leptin and leptin binding in the human circulation. Ann Clin Biochem. 2000;37:244–52.
Rutkowski JM, Scherer PE. Isolation and quantitation of adiponectin higher order complexes. Methods Enzymol. 2014;537:243–59.
Martin RM, Patel R, Zinovik A, Kramer MS, Oken E, Vilchuck K, et al. Filter Paper Blood Spot Enzyme Linked Immunoassay for Insulin and Application in the Evaluation of Determinants of Child Insulin Resistance. PLoS ONE. 2012;7:e46752.
Hosoda H, Kangawa K. Ghrelin Measurement: Present and Perspectives. In: Ghigo E, Benso A, Broglio F (eds). Ghrelin. Kluwer Academic Publishers: Boston, 2004, pp 225-36.
CDC. Disability and Obesity | CDC. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/obesity.html (accessed 20 Sep 2024).
Anscombe FJ, Barron BA. Treatment of outliers in samples of size three. J RES NATL BUR STAN SECT B MATH MATH PHYS. 1966;70B:141.
Molenberghs G, Beunckens C, Sotto C, Kenward MG. Every Missingness not at Random Model Has a Missingness at Random Counterpart with Equal Fit. J R Stat Soc Ser B: Stat Methodol. 2008;70:371–88.
Rubin DB. Inference and missing data. Biometrika. 1976;63:581–92.
Erler NS, Rizopoulos D, Lesaffre EMEH. JointAI: Joint Analysis and Imputation of Incomplete Data in R. J Stat Softw. 2021;100:1–56.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. https://www.r-project.org/.
Donato JJ. Programming of metabolism by adipokines during development. Nat Rev Endocrino. 2023;19:385–97.
Schubring C, Siebler T, Kratzsch J, Englaro P, Blum WF, Triep K, et al. Leptin serum concentrations in healthy neonates within the first week of life: relation to insulin and growth hormone levels, skinfold thickness, body mass index and weight. Clin Endocrinol (Oxf). 1999;51:199–204.
Martos-Moreno GÁ, Barrios V, Sáenz de Pipaón M, Pozo J, Dorronsoro I, Martínez-Biarge M, et al. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: relationship with glucose metabolism. Eur J Endocrinol. 2009;161:381–9.
Ertl T, Funke S, Sárkány I, Szabó I, Rascher W, Blum WernerF, et al. Postnatal changes of leptin levels in full-term and preterm neonates: their relation to intrauterine growth, gender and testosterone. Biol Neonate. 1999;75:167–76.
Buck CO, Li N, Eaton CB, Kelsey KT, Cecil KM, Kalkwarf HJ, et al. Neonatal and adolescent adipocytokines as predictors of adiposity and cardiometabolic risk in adolescence. Obes (Silver Spring). 2021;29:1036–45.
Siahanidou T, Mandyla H, Papassotiriou G-P, Papassotiriou I, Chrousos G. Circulating levels of adiponectin in preterm infants. Arch Dis Child - Fetal Neonatal Ed. 2007;92:F286–F290.
Yoshida T, Nagasaki H, Asato Y, Ohta T. The ratio of high-molecular weight adiponectin and total adiponectin differs in preterm and term infants. Pediatr Res. 2009;65:580–3.
Han L, Li B, Xu X, Liu S, Li Z, Li M, et al. Umbilical cord blood adiponectin, leptin, insulin, and ghrelin in premature infants and their association with birth outcomes. Front Endocrinol. 2021; 12.https://www.frontiersin.org/articles/10.3389/fendo.2021.738964 (accessed 4 Apr 2023).
Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130:e640–649.
Devaskar SU, Chu A. Intrauterine growth restriction: hungry for an answer. Physiology. 2016;31:131–46.
Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol-Endocrinol Metab. 2013;304:E516–E523.
Frost MS, Zehri AH, Limesand SW, Hay WW, Rozance PJ. Differential effects of chronic pulsatile versus chronic constant maternal hyperglycemia on fetal pancreatic β -cells. J Pregnancy. 2012;2012:1–8.
Gray IP, Cooper PA, Cory BJ, Toman M, Crowther NJ. The intrauterine environment is a strong determinant of glucose tolerance during the neonatal period, even in prematurity. J Clin Endocrinol Metab. 2002;87:4252–6.
Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853–70.
Cho GJ, Yoo SW, Hong SC, Oh M-J, Kim T, Kim HJ, et al. Correlations between umbilical and maternal serum resistin levels and neonatal birth weight. Acta Obstetricia et Gynecologica Scand. 2006;85:1051–6.
Nava-Salazar S, Flores-Pliego A, Pérez-Martínez G, Parra-Hernández S, Vanoye-Carlo A, Ibarguengoitia-Ochoa F, et al. Resistin modulates low-density lipoprotein cholesterol uptake in human placental explants via PCSK9. Reprod Sci. 2022;29:3242–53.
Savino F, Lupica MM, Liguori SA, Fissore MF, Silvestro L. Ghrelin and feeding behaviour in preterm infants. Early Hum Dev. 2012;88:S51–S55.
Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. a clinical review. Int J Mol Sci. 2021;22:2965.
Bellone S, Baldelli R, Radetti G, Rapa A, Vivenza D, Petri A, et al. Ghrelin secretion in preterm neonates progressively increases and is refractory to the inhibitory effect of food intake. J Clin Endocrinol Metab. 2006;91:1929–33.
Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol. 2017;13:50–62.
Ng PC, Lee CH, Lam CWK, Chan IHS, Wong E, Fok TF. Ghrelin in preterm and term newborns: relation to anthropometry, leptin and insulin. Clin Endocrinol. 2005;63:217–22.
Shimizu T, Kitamura T, Yoshikawa N, Suganuma H, Hisata K, Tanaka K, et al. Plasma levels of active ghrelin until 8 weeks after birth in preterm infants: relationship with anthropometric and biochemical measures. Arch Dis Child Fetal Neonatal Ed. 2007;92:F291–F292.
Lis-Kuberka J, Pupek M, Orczyk-Pawiłowicz M. The mother-child dyad adipokine pattern: a review of current knowledge. Nutrients. 2023;15:4059.
Acknowledgements
We would like to thank the Yale Neonatal NOuRISH Research Team at Yale School of Medicine for their work in enrolling subjects in this study and their involvement in the conduction of body composition assessments for subjects enrolled in this study.
Funding
This publication was in part made possible by CTSA Grant Number UL1TR001863 from NCATS, a component of the NIH; the COVID-19 Fund to Retain Clinical Scientists at Yale, sponsored by the Doris Duke Charitable Foundation award #2021266, and the Yale Center for Clinical Investigation; the Robert E. Leet and Clara Guthrie Patterson Trust Mentored Research Award, Bank of America, Private Bank, Trustee; and the Society for Pediatric Research Bridging to Success Award. Research reported in this publication was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T35DK104689. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Study conceptualization, COB, CGB, SNT; Methodology, COB, VS; Formal Analysis CGB, VS; Investigation, COB; Writing – Original Draft Preparation, CGB; Writing – Review & Editing, COB, VS, SNT; Supervision, COB, SNT; Funding Acquisition, COB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in accordance with the declaration of Helsinki and was approved by the Institutional Review Board at Yale University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borden, C.G., Shabanova, V., Taylor, S.N. et al. Alterations in infant adipokine concentrations in the first postnatal week with exposure to diabetes in pregnancy. J Perinatol 45, 622–627 (2025). https://doi.org/10.1038/s41372-025-02269-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02269-2