Abstract
Objectives
To study the effect of hypothermia on surfactant proteins, anti-inflammatory and pro-fibrotic mediators
Design
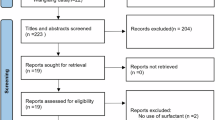
Prospective, pragmatic study enrolling asphyxiated neonates without lung injury. Surfactant proteins (SP), Club cell secretory protein (CC-10), tumor growth factor-β1 (TGF-β1), human fibroblast growth factor-2 (hFGF) and anti-inflammatory interleukins (IL) were measured in broncho-alveolar lavage fluids obtained before, during and after hypothermia.
Results
Twelve neonates were studied. SP-A, -B, -C, -D and CC-10 levels were similar before, during and after hypothermia. IL-10, IL-13, IL-14 were inconsistently detected only in five out of twelve patients and not in all timepoints; TGF-β1 was always undetectable. hFGF decreased during hypothermia (pre = 92 [52–193.6], during = 19.4 [0–42.6], post = 31.8 [0–83] ng/mL, p = 0.026) with levels before hypothermia being significantly higher than those obtained during and after the treatment (post hoc p = 0.025).
Conclusions
In cooled neonates without any lung disorder, hypothermia is associated with lower hFGF but not with any changes in surfactant proteins or anti-inflammatory molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The deidentified datasets are available from the corresponding author on reasonable request for research purposes and provided that all relevant regulations are respected.
References
Pietrini D, Piastra M, Luca E, Mancino A, Conti G, Cavaliere F, et al. Neuroprotection and hypothermia in infants and children. Curr Drug Targets. 2012;13:925–35.
American Academy of Pediatrics. Committee on Fetus and Newborn, Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146–50. https://doi.org/10.1542/peds.2014-0899
Hong SB, Koh Y, Lee IC, Kim MJ, Kim WS, Kim DS, et al. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Crit Care Med. 2005;33:2049–55. https://doi.org/10.1097/01.CCM.0000178186.37167.53
Huang P-S, Tang G-J, Chen C-H, Kou YR. Whole-body moderate hypothermia confers protection from wood smoke-induced acute lung injury in rats: The therapeutic window. Crit Care Med. 2006;34:1160–7. https://doi.org/10.1097/01.CCM.0000207342.50559.0F
Ball MK, Hillman NH, Kallapur SG, Polglase GR, Jobe AH, Pillow JJ. Body temperature effects on lung injury in ventilated preterm lambs. Resuscitation. 2010;81:749–54. https://doi.org/10.1016/j.resuscitation.2009.12.007
Cruces P, Erranz B, Donoso A, Carvajal C, Salomón T, Torres MF, et al. Mild hypothermia increases pulmonary anti-inflammatory response during protective mechanical ventilation in a piglet model of acute lung injury. Pediatr Anesth. 2013;23:1069–77. https://doi.org/10.1111/pan.12209
Angus SA, Henderson WR, Banoei MM, Molgat-Seon Y, Peters CM, Parmar HR, et al. Therapeutic hypothermia attenuates physiologic, histologic, and metabolomic markers of injury in a porcine model of acute respiratory distress syndrome. Physiol Rep. 2022;10:e15286. https://doi.org/10.14814/phy2.15286
Akyol O, Demirgan S, Şengelen A, Güneyli HC, Oran DS, Yıldırım F, et al. Mild Hypothermia via External Cooling Improves Lung Function and Alleviates Pulmonary Inflammatory Response and Damage in Two-Hit Rabbit Model of Acute Lung Injury. J Invest Surg. 2022;35:1472–83. https://doi.org/10.1080/08941939.2022.2064010
De Luca D, Shankar-Aguilera S, Autilio C, Raschetti R, Vedovelli L, Fitting C, et al. Surfactant-secreted phospholipase A 2 interplay and respiratory outcome in preterm neonates. Am J Physiol Lung Cell Mol Physiol. 2020;319:L95–104. https://doi.org/10.1152/ajplung.00462.2019
De Luca D, Vázquez-Sánchez S, Minucci A, Echaide M, Piastra M, Conti G, et al. Effect of whole-body hypothermia on inflammation and surfactant function in asphyxiated neonates. Eur Resp J. 2014;44:1708–10. https://doi.org/10.1183/09031936.00117714
Autilio C, Shankar-Aguilera S, Minucci A, Touqui L, De Luca D. Effect of cooling on lung secretory phospholipase A2 activity in vitro, ex vivo, and in vivo. Am J Physiol Lung Cell Mol Physiol. 2019;316:L498–505. https://doi.org/10.1152/ajplung.00201.2018
Langman C, Orgeig S, Daniels CB. Alterations in composition and function of surfactant associated with torpor in Sminthopsis crassicaudata. Am J Physiol. 1996;271:R437–45. https://doi.org/10.1152/ajpregu.1996.271.2.R437
Suri LN, McCaig L, Picardi MV, Ospina OL, Veldhuizen RA, Staples JF, et al. Adaptation to low body temperature influences pulmonary surfactant composition thereby increasing fluidity while maintaining appropriately ordered membrane structure and surface activity. Biochim Biophys Acta. 2012;1818:1581–9. https://doi.org/10.1016/j.bbamem.2012.02.021
Autilio C, Echaide M, Cruz A, García-Mouton C, Hidalgo A, Da Silva E, et al. Molecular and biophysical mechanisms behind the enhancement of lung surfactant function during controlled therapeutic hypothermia. Sci Rep. 2021;11:728. https://doi.org/10.1038/s41598-020-79025-3
Autilio C, Echaide M, Dell’Orto V, Perez-Gil J, De Luca D. Effect of Whole Body Hypothermia on Surfactant Function When Amniotic Fluid is Meconium Stained. Ther Hypothermia Temp Manag. 2020;10:186–89. https://doi.org/10.1089/ther.2017.0012
Autilio C, Echaide M, De Luca D, Perez-Gil J. Controlled hypothermia may improve surfactant function in asphyxiated neonates with or without meconium aspiration syndrome. PLoS One. 2018;13:e0192295. https://doi.org/10.1371/journal.pone.0192295
Nespeca M, Giorgetti C, Nobile S, Ferrini I, Simonato M, Verlato G, et al. Does Whole-Body Hypothermia in Neonates with Hypoxic–Ischemic Encephalopathy Affect Surfactant Disaturated-Phosphatidylcholine Kinetics? PLoS One. 2016;11:e0153328. https://doi.org/10.1371/journal.pone.0153328
Hayek AJ, White HD, Ghamande S, Spradley C, Arroliga AC. Is Therapeutic Hypothermia for Acute Respiratory Distress Syndrome the Future? J Intensive Care Med. 2017;32:460–4. https://doi.org/10.1177/0885066617701117
Pietrini D, Pennisi M, Vitale F, Pulitanò SM, Conti G, Mancino A, et al. Rescue hypothermia for refractory hypercapnia. Eur J Pediatr. 2012;171:1855–7. https://doi.org/10.1007/s00431-012-1769-6
Cruces P, Cores C, Casanova D, Pizarro F, Diaz F. Successful use of mild therapeutic hypothermia as compassionate treatment for severe refractory hypoxemia in COVID-19. J Crit Care. 2021;63:260–3. https://doi.org/10.1016/j.jcrc.2021.01.008
Karnatovskaia LV, Festic E, Freeman WD, Lee AS. Effect of Therapeutic Hypothermia on Gas Exchange and Respiratory Mechanics: A Retrospective Cohort Study. Ther Hypothermia Temp Manag. 2014;4:88–95. https://doi.org/10.1089/ther.2014.0004
Slack DF, Corwin DS, Shah NG, Shanholtz CB, Verceles AC, Netzer G, et al. Pilot Feasibility Study of Therapeutic Hypothermia for Moderate to Severe Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45:1152–9. https://doi.org/10.1097/CCM.0000000000002338
Aslami H, Binnekade JM, Horn J, Huissoon S, Juffermans NP. The effect of induced hypothermia on respiratory parameters in mechanically ventilated patients. Resuscitation. 2010;81:1723–5. https://doi.org/10.1016/j.resuscitation.2010.09.006
De Luca D, Tingay DG, van Kaam A, Brunow de Carvalho W, Valverde E, Christoph Roehr C, et al. Hypothermia and Meconium Aspiration Syndrome: International Multicenter Retrospective Cohort Study. Am J Resp Crit Care Med. 2016;194:381–4. https://doi.org/10.1164/rccm.201602-0422LE
https://clinicaltrials.gov/study/NCT04545424 n.d. [Accessed on Sept 30, 2024]
De Luca D, Capoluongo E, Rigo V. Study group on Secretory Phospholipase in Paediatrics (SSPP). Secretory phospholipase A2 pathway in various types of lung injury in neonates and infants: a multicentre translational study. BMC Pediatr. 2011;11:101. https://doi.org/10.1186/1471-2431-11-101.
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58.
De Luca D, Minucci A, Tripodi D, Piastra M, Pietrini D, Zuppi C, et al. Role of distinct phospholipases A2 and their modulators in meconium aspiration syndrome in human neonates. Intensive Care Med. 2011;37:1158–65. https://doi.org/10.1007/s00134-011-2243-z
de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, et al. On behalf of the ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Bronchoalveolar lavage in children. Eur Resp J. 2000;15:217–31. https://doi.org/10.1183/09031936.00.15121700
Dell’Orto V, Bourgeois-Nicolaos N, Rouard C, Romain O, Shankar-Aguilera S, Doucet-Populaire F, et al. Cell Count Analysis from Nonbronchoscopic Bronchoalveolar Lavage in Preterm Infants. J Pediatr. 2018;200:30–7.e2. https://doi.org/10.1016/j.jpeds.2018.04.074
McNeil JB, Shaver CM, Kerchberger VE, Russell DW, Grove BS, Warren MA, et al. Novel Method for Noninvasive Sampling of the Distal Airspace in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2018;197:1027–35. https://doi.org/10.1164/rccm.201707-1474OC
Autilio C, Echaide M, Benachi A, Marfaing-Koka A, Capoluongo ED, Pérez-Gil J, et al. A Noninvasive Surfactant Adsorption Test Predicting the Need for Surfactant Therapy in Preterm Infants Treated with Continuous Positive Airway Pressure. J Pediatr. 2017;182:66–73.e1. https://doi.org/10.1016/j.jpeds.2016.11.057
Autilio C, Echaide M, Benachi A, Marfaing-Koka A, Capoluongo ED, Pérez-Gil J, et al. Surfactant Injury in the Early Phase of Severe Meconium Aspiration Syndrome. Am J Respir Cell Mol Biol. 2020;63:327–37. https://doi.org/10.1165/rcmb.2019-0413OC
Griese M, Kirmeier HG, Liebisch G, Rauch D, Stückler F, Schmitz G, et al. ILD-BAL working group of the Kids-Lung-Register. Surfactant lipidomics in healthy children and childhood interstitial lung disease. PLoS One. 2015;10:e0117985. https://doi.org/10.1371/journal.pone.0117985
Liu M, Zhang J, Liu C. Clinical efficacy of recombinant human latrophilin 3 antibody in the treatment of pediatric asthma. Exp Ther Med. 2018;15:539–47.
Cook DB, McLucas BC, Montoya LA, Brotski CM, Das S, Miholits M, et al. Multiplexing protein and gene level measurements on a single Luminex platform. Methods. 2019;158:27–32. https://doi.org/10.1016/j.ymeth.2019.01.018
De Luca D, Lopez-Rodriguez E, Minucci A, Vendittelli F, Gentile L, Stival E, et al. Clinical and biological role of secretory phospholipase A2 in acute respiratory distress syndrome infants. Crit Care. 2013;17:R163. https://doi.org/10.1186/cc12842
D’Aronco S, Simonato M, Vedovelli L, Baritussio A, Verlato G, Nobile S, et al. Surfactant protein B and A concentrations are increased in neonatal pneumonia. Pediatr Res. 2015;78:401–6.
Autilio C, Perez-Gil J. Understanding the principle biophysics concepts of pulmonary surfactant in health and disease. Arch Dis Child Fetal Neonatal Ed. 2019;104:F443–51.
Tokieda K, Ikegami M, Wert SE, Baatz JE, Zou Y, Whitsett JA. Surfactant Protein B Corrects Oxygen-Induced Pulmonary Dysfunction in Heterozygous Surfactant Protein B–Deficient Mice. Pediatr Res. 1999;46:708–14. https://doi.org/10.1203/00006450-199912000-00014
Chaby R, Garcia-Verdugo I, Espinassous Q, Augusto LA. Interactions between LPS and lung surfactant proteins. J Endotoxin Res. 2005;11:181–5. https://doi.org/10.1179/096805105X37358
Roldan N, Nyholm TKM, Slotte JP, Perez-Gil J, Garcia-Álvarez B. Effect of Lung Surfactant Protein SP-C and SP-C-Promoted Membrane Fragmentation on Cholesterol Dynamics. Biophys J. 2016;111:1703–13. https://doi.org/10.1016/j.bpj.2016.09.016
Arbibe L, Koumanov K, Vial D, Rougeot C, Faure G, Havet N, et al. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest. 1998;102:1152–60. https://doi.org/10.1172/JCI3236
Arroyo R, Khan MA, Echaide M, Perez-Gil J, Palaniyar N. SP-D attenuates LPS-induced formation of human neutrophil extracellular traps (NETs), protecting pulmonary surfactant inactivation by NETs. Commun Biol. 2019;2:470. https://doi.org/10.1038/s42003-019-0662-5
Martinu T, Todd JL, Gelman AE, Guerra S, Palmer SM. Club Cell Secretory Protein in Lung Disease: Emerging Concepts and Potential Therapeutics. Annu Rev Med. 2023;74:427–41. https://doi.org/10.1146/annurev-med-042921-123443
Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le Bourhis X. FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev. 2000;11:295–302.
Presta M, Andres G, Leali D, Dell’Era P, Ronca R. Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur Cytokine Netw. 2009;20:39–50. https://doi.org/10.1684/ecn.2009.0155
El Agha E, Seeger W, Bellusci S. Therapeutic and pathological roles of fibroblast growth factors in pulmonary diseases. Dev Dyn. 2017;246:235–44. https://doi.org/10.1002/dvdy.24468
Dargaville PA, South M, McDougall PN. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am J Respir Crit Care Med. 1999;160:771–7. https://doi.org/10.1164/ajrccm.160.3.9811048
Author information
Authors and Affiliations
Contributions
CA: manuscript drafting, formal analysis, investigation, data curation. LT: laboratory assays, data interpretation, resources. SF: investigation, laboratory assays, resources. RA: investigation, laboratory assays, data curation, resources. PSK: investigation, data curation, resources. AAA: investigation, data curation, resources. JPG: investigation, validation, resources. DDL: study conception, manuscript drafting, formal analysis, methodology, resources. All authors critically revised the manuscript for important intellectual content and approved the final manuscript version to be published.
Corresponding author
Ethics declarations
Competing interests
PSK served as Chief Medical Officer from 2018-2021 for Airway Therapeutics inc. which is developing SPD as a human therapeutic agent; all his financial relationships with Airway Therapeutics inc. terminated in 2021. RA is currently an employee of Airway Therapeutics inc. JPG and DDL received research grants or consultancy fees from Airway Therapeutics inc. This company had no role whatsoever in design, preparation and conduction or review of the work, neither in the approval of the manuscript or decision to submit it for publication. The declared interests are all unrelated to the present work. The other authors have no conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Autilio, C., Touqui, L., Foligno, S. et al. Effect of therapeutic hypothermia on surfactant proteins, anti-inflammatory and pro-fibrotic mediators. J Perinatol 45, 1058–1063 (2025). https://doi.org/10.1038/s41372-025-02285-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02285-2