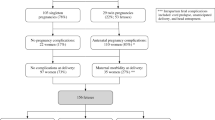
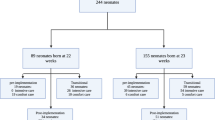
Abstract
Preterm birth between 22 and 25 5/7 weeks complicates <1% of live births within the United States though contributes more than 20% of infant mortality within the first year of life. Ante- and intrapartum interventions such as antenatal corticosteroids, magnesium sulfate, and tocolytic and antibiotic therapies have been shown effective in optimizing postnatal prognosis in births at 24 weeks and beyond. Interventions, mode of delivery, and resuscitation plans should ideally be discussed with the perinatology, neonatology, and nursing teams with the family using shared decision making. Observational data have alluded to similar postnatal benefits in births at 22–23 weeks; however, these data are limited by small sample sizes, inconsistencies in outcome reporting, and variations in management strategies. Future studies to evaluate the utility of these interventions among births at 22–23 weeks are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. National institute of child health and human development neonatal research network. Intensive care for extreme prematurity–moving beyond gestational age. N. Engl J Med. 2008;358:1672–81. https://doi.org/10.1056/NEJMoa073059.
Seasely AR, Jauk VC, Szychowski JM, Ambalavanan N, Tita AT, Casey BM. Maternal and neonatal outcomes at periviable gestation throughout delivery admission. Am J Perinatol. 2024;41:e2952–e2958. https://doi.org/10.1055/s-0043-1776347.
Draper ES, Gallimore ID, Smith LK, et al. MBRRACE-UK perinatal mortality surveillance report: UK perinatal deaths for births from January to December 2019. Infant Mortality Morbidity Stud. 2021. [MBRRACE-UK_Perinatal_Surveillance_Report_2020.pdf].
Ancel PY, Goffinet F, EPIPAGE-2 Writing Group, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015;169:230–8. https://doi.org/10.1001/jamapediatrics.2014.3351. Erratum in: JAMA Pediatr. 2015 Apr;169(4):323. https://doi.org/10.1001/jamapediatrics.2015.0528. Alberge, Catherine [Corrected to Alberge, Corine].
Morgan AS, Zeitlin J, Källén K, Draper ES, Maršál K, Norman M, et al. Birth outcomes between 22 and 26 weeks’ gestation in national population-based cohorts from Sweden, England and France. Acta Paediatr. 2022;111:59–75. https://doi.org/10.1111/apa.16084.
Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402:1261–71. https://doi.org/10.1016/S0140-6736(23)00878-4. Erratum in: Lancet. 2024 Feb 17;403(10427):618. doi: 10.1016/S0140-6736(24)00267-8.
MacDorman MF, Matthews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital–Stat Rep. 2014;63:1–6.
Woods CR, Davis DW, Duncan SC, Myers JA, O’Shea TM. Variation in classification of live birth with newborn period death versus fetal death at the local level may impact reported infant mortality rate. BMC Pediatr. 2014;14:108.
Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Fetal Deaths on CDC WONDER Online Database. Data are from the Fetal Death Records 2014-2022, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/fetal-deaths-expanded-current.html on Nov 24, 2024 9:32:41 AM.
Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Natality on CDC WONDER Online Database. Data are from the Natality Records 2016-2023, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/natality-expanded-current.html on Nov 24, 2024 9:33:08 AM.
Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022 6:106–115. https://doi.org/10.1016/S2352-4642(21)00311-4. Epub 2021 Nov 17. Erratum in: Lancet Child Adolesc Health. 2022 Jan;6(1):e4. Doi: 10.1016/S2352-4642(21)00382-5.
Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Linked Birth / Infant Deaths on CDC WONDER Online Database. Data are from the Linked Birth / Infant Deaths Records 2017-2021, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/lbd-current-expanded.html on Jul 31, 2024 12:36:24 PM.
Tyson JE, Stoll BJ. Evidence-based ethics and the care and outcome of extremely premature infants. Clin Perinatol. 2003;30:363–87. https://doi.org/10.1016/s0095-5108(03)00028-9. PMID: 12875360
Mactier H, Bates SE, Johnston T, Lee-Davey C, Marlow N, Mulley K, et al. BAPM Working Group. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:232–9. https://doi.org/10.1136/archdischild-2019-318402.
Cummings J. COMMITTEE ON FETUS AND NEWBORN. Antenatal counseling regarding resuscitation and intensive care before 25 weeks of gestation. Pediatrics. 2015;136:588–95. https://doi.org/10.1542/peds.2015-2336.
Ambalavanan N, Carlo WA, Tyson JE, Langer JC, Walsh MC, Parikh NA, et al. Generic Database; Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Outcome trajectories in extremely preterm infants. Pediatrics. 2012;130:e115–25. https://doi.org/10.1542/peds.2011-3693.
Skupski DW, McCullough LB, Levene M, Chervenak FA. Improving obstetric estimation of outcomes of extremely premature neonates: an evolving challenge. J Perinat Med. 2010;38:19–22. https://doi.org/10.1515/jpm.2010.013.
Boland RA, Cheong JLY, Stewart MJ, Kane SC, Doyle LW. Disparities between perceived and true outcomes of infants born at 23-25 weeks’ gestation. Aust N. Z J Obstet Gynaecol. 2022;62:255–62. https://doi.org/10.1111/ajo.13443.
Itabashi K, Miyazawa T, Kusuda S, Wada K. Japan Pediatric Society Newborn Committee. Changes in mortality rates among extremely preterm infants born before 25 weeks’ gestation: Comparison between the 2005 and 2010 nationwide surveys in Japan. Early Hum Dev. 2021;155:105321 https://doi.org/10.1016/j.earlhumdev.2021.105321.
Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321:1188–99. https://doi.org/10.1001/jama.2019.2021. Erratum in: JAMA. 2024 Jan 16;331(3):262. https://doi.org/10.1001/jama.2023.26372.
Farooqi A, Hakansson S, Serenius F, Kallen K, Björklund L, Normann E, et al. One-year survival and outcomes of infants born at 22 and 23 weeks of gestation in Sweden 2004-2007, 2014-2016 and 2017-2019. Arch Dis Child Fetal Neonatal Ed. 2023;109:10–17. https://doi.org/10.1136/archdischild-2022-325164.
Isayama T, Miyakoshi K, Namba F, Hida M, Morioka I, Ishii K, et al. Survival and unique clinical practices of extremely preterm infants born at 22-23 weeks’ gestation in Japan: a national survey. Arch Dis Child Fetal Neonatal Ed. 2024;110:17–22. https://doi.org/10.1136/archdischild-2023-326355.
de Laat MW, Wiegerinck MM, Walther FJ, Boluyt N, Mol BW, van der Post JA, et al. Nederlandse Vereniging voor Kindergeneeskunde; Nederlandse Vereniging voor Obstetrie en Gynaecologie. Richtlijn ‘Perinataal beleid bij extreme vroeggeboorte’ [Practice guideline ‘Perinatal management of extremely preterm delivery’]. Ned Tijdschr Geneeskd. 2010;154:A2701. Dutch.
Gordon HG, Shub A, Stewart MJ, Kane SC, Cheong JL, Roberts CT, et al. In-utero transfer, survival-focused care and survival to 28-days at 22-24 weeks’ gestation pre- and post- implementation of an extreme prematurity management guideline in Victoria, Australia. BMJ Paediatr Open. 2024;8:e002462 https://doi.org/10.1136/bmjpo-2023-002462.
Nair Shah N, Krishna I, Vyas-Read S, Patel RM. Neonatal and obstetric provider perceptions and management at 22 weeks’ gestation. Am J Perinatol. 2024;41:e879–e885. https://doi.org/10.1055/a-1969-1237.
Tucker Edmonds B, McKenzie F, Farrow V, Raglan G, Schulkin J. A national survey of obstetricians’ attitudes toward and practice of periviable intervention. J Perinatol. 2015;35:338–43. https://doi.org/10.1038/jp.2014.201.
WHO ACTION Trials Collaborators, Oladapo OT, Vogel JP, Piaggio G, Nguyen MH, Althabe F, et al. Antenatal dexamethasone for early preterm birth in low-resource countries. N Engl J Med. 2020;383:2514–25. https://doi.org/10.1056/NEJMoa2022398.
Abiramalatha T, Bandyopadhyay T, Ramaswamy VV, Shaik NB, Thanigainathan S, Pullattayil AK, et al. Risk factors for periventricular leukomalacia in preterm infants: a systematic review, meta-analysis, and GRADE-based assessment of certainty of evidence. Pediatr Neurol. 2021;124:51–71. https://doi.org/10.1016/j.pediatrneurol.2021.08.003.
Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. Br J Obstet Gynaecol. 1989;96:401–10. https://doi.org/10.1111/j.1471-0528.1989.tb02413.x.
Papageorgiou AN, Desgranges MF, Masson M, Colle E, Shatz R, Gelfand MM. The antenatal use of betamethasone in the prevention of respiratory distress syndrome: a controlled double-blind study. Pediatrics. 1979;63:73–9.
Herrera TI, Vaz Ferreira MC, Toso A, Villarroel L, Silvera F, Ceriani-Cernadas JM, et al. Neocosur neonatal network. neonatal outcomes of antenatal corticosteroids in preterm multiple pregnancies compared to singletons. Early Hum Dev. 2019;130:44–50. https://doi.org/10.1016/j.earlhumdev.2019.01.008.
Travers CP, Clark RH, Spitzer AR, Das A, Garite TJ, Carlo WA. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: prospective cohort study. BMJ. 2017;356:j1039 https://doi.org/10.1136/bmj.j1039.
McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12:CD004454 https://doi.org/10.1002/14651858.CD004454.pub4.
Ehret DEY, Edwards EM, Greenberg LT, Bernstein IM, Buzas JS, Soll RF, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1:e183235 https://doi.org/10.1001/jamanetworkopen.2018.3235.
Chawla S, Wyckoff MH, Rysavy MA, Patel RM, Chowdhury D, Natarajan G, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of Antenatal Steroid Exposure at 21 to 22 Weeks of Gestation With Neonatal Survival and Survival Without Morbidities. JAMA Netw Open. 2022;5:e2233331 https://doi.org/10.1001/jamanetworkopen.2022.33331.
Mori R, Kusuda S, Fujimura M. Neonatal Research Network Japan. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011;159:110–.e1. https://doi.org/10.1016/j.jpeds.2010.12.039.
Travers CP, Carlo WA, McDonald SA, Das A, Bell EF, Ambalavanan N, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018;218:130.e1–130.e13. https://doi.org/10.1016/j.ajog.2017.11.554.
Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. https://doi.org/10.1001/jama.2011.1752.
Hayes EJ, Paul DA, Stahl GE, Seibel-Seamon J, Dysart K, Leiby BE, et al. Effect of antenatal corticosteroids on survival for neonates born at 23 weeks of gestation. Obstet Gynecol. 2008;111:921–6. https://doi.org/10.1097/AOG.0b013e318169ce2d.
Yim CL, Tam M, Chan HL, Tang SM, Au SCL, Yip WWK, et al. Association of antenatal steroid and risk of retinopathy of prematurity: a systematic review and meta-analysis. Br J Ophthalmol. 2018;102:1336–41. https://doi.org/10.1136/bjophthalmol-2017-311576.
Sacco A, Cornish EF, Marlow N, David AL, Giussani DA. The effect of antenatal corticosteroid use on offspring cardiovascular function: A systematic review. BJOG. 2023;130:325–33. https://doi.org/10.1111/1471-0528.17316.
Walters AGB, Gamble GD, Crowther CA, Dalziel SR, Eagleton CL, McKinlay CJD, et al. Cardiovascular outcomes 50 years after antenatal exposure to betamethasone: Follow-up of a randomised double-blind, placebo-controlled trial. PLoS Med. 2024;21:e1004378 https://doi.org/10.1371/journal.pmed.1004378.
Crowther CA, Anderson PJ, McKinlay CJ, Harding JE, Ashwood PJ, Haslam RR, et al. ACTORDS Follow-up Group. Mid-childhood outcomes of repeat antenatal corticosteroids: a randomized controlled trial. Pediatrics. 2016;138:e20160947 https://doi.org/10.1542/peds.2016-0947.
Gentle SJ, Carlo WA, Tan S, Gargano M, Ambalavanan N, Chawla S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network. Association of Antenatal Corticosteroids and Magnesium Sulfate Therapy With Neurodevelopmental Outcome in Extremely Preterm Children. Obstet Gynecol. 2020;135:1377–86. https://doi.org/10.1097/AOG.0000000000003882.
Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:1385–96. https://doi.org/10.1097/AOG.0000000000000748.
Salokorpi T, Sajaniemi N, Hällback H, Kari A, Rita H, von Wendt L. Randomized study of the effect of antenatal dexamethasone on growth and development of premature children at the corrected age of 2 years. Acta Paediatr. 1997;86:294–8. https://doi.org/10.1111/j.1651-2227.1997.tb08893.x.
Ciapponi A, Klein K, Colaci D, Althabe F, Belizán JM, Deegan A, et al. Dexamethasone versus betamethasone for preterm birth: a systematic review and network meta-analysis. Am J Obstet Gynecol MFM. 2021;3:100312 https://doi.org/10.1016/j.ajogmf.2021.100312.
Williams MJ, Ramson JA, Brownfoot FC. Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth. Cochrane Database Syst Rev. 2022;8:CD006764 https://doi.org/10.1002/14651858.CD006764.pub4.
Elimian A, Garry D, Figueroa R, Spitzer A, Wiencek V, Quirk JG. Antenatal betamethasone compared with dexamethasone (betacode trial): a randomized controlled trial. Obstet Gynecol. 2007;110:26–30. https://doi.org/10.1097/01.AOG.0000268281.36788.81.
Crowther CA, Ashwood P, Andersen CC, Middleton PF, Tran T, Doyle LW, et al. ASTEROID Study Group. Maternal intramuscular dexamethasone versus betamethasone before preterm birth (ASTEROID): a multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health. 2019;3:769–80. https://doi.org/10.1016/S2352-4642(19)30292-5.
Chawanpaiboon S, Chukaew R, Pooliam J. A comparison of 2 doses of antenatal dexamethasone for the prevention of respiratory distress syndrome: an open-label, noninferiority, pragmatic randomized trial. Am J Obstet Gynecol. 2024;230:260.e1–260.e19. https://doi.org/10.1016/j.ajog.2023.07.006.
Schmitz T, Doret-Dion M, Sentilhes L, Parant O, Claris O, Renesme L, et al. BETADOSE trial study group; Groupe de Recherche en Obstétrique et Gynécologie. Neonatal outcomes for women at risk of preterm delivery given half dose versus full dose of antenatal betamethasone: a andomized, multicentre, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2022;400:592–604. https://doi.org/10.1016/S0140-6736(22)01535-5. Erratum in: Lancet. 2022 Oct 22;400(10361):1404. Doi: 10.1016/S0140-6736(22)01696-8.
Battarbee AN, Ros ST, Esplin MS, Biggio J, Bukowski R, Parry S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Genomics and Proteomics Network for Preterm Birth Research (GPN-PBR). Optimal timing of antenatal corticosteroid administration and preterm neonatal and early childhood outcomes. Am J Obstet Gynecol MFM. 2020;2:100077 https://doi.org/10.1016/j.ajogmf.2019.100077.
Norman M, Piedvache A, Børch K, Huusom LD, Bonamy AE, Howell EA, et al. Effective Perinatal Intensive Care in Europe (EPICE) Research Group. Association of Short Antenatal Corticosteroid Administration-to-Birth Intervals With Survival and Morbidity Among Very Preterm Infants: Results From the EPICE Cohort. JAMA Pediatr. 2017;171:678–86. https://doi.org/10.1001/jamapediatrics.2017.0602.
Norman J, Shennan A, Jacobsson B, Stock SJ. FIGO Working Group for Preterm Birth. FIGO good practice recommendations on the use of prenatal corticosteroids to improve outcomes and minimize harm in babies born preterm. Int J Gynaecol Obstet. 2021;155:26–30. https://doi.org/10.1002/ijgo.13836. Erratum in: Int J Gynaecol Obstet. 2022 May;157(2):486. https://doi.org/10.1002/ijgo.14145. PMID: 34520057.
Abbasalizadeh F, Pouya K, Zakeri R, Asgari-Arbat R, Abbasalizadeh S, Parnianfard N. Prenatal administration of betamethasone and neonatal respiratory distress syndrome in multifetal pregnancies: a randomized controlled trial. Curr Clin Pharm. 2020;15:164–9. https://doi.org/10.2174/1574884714666191007154936.
Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. 2023;40:1789–97. https://doi.org/10.1055/s-0041-1740062.
Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95:263–9.
Crowther CA, Hiller JE, Doyle LW, Haslam RR, Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–76. https://doi.org/10.1001/jama.290.20.2669.
Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. Eunice Kennedy Shriver NICHD maternal-fetal medicine units network. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N. Engl J Med. 2008;359:895–905. https://doi.org/10.1056/NEJMoa0801187.
Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. AMICABLE Group. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med. 2017;14:e1002398 https://doi.org/10.1371/journal.pmed.1002398.
Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, et al. PREMAG trial group. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG. 2007;114:310–8. https://doi.org/10.1111/j.1471-0528.2006.01162.x.
Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–8. https://doi.org/10.1067/mob.2002.123544.
Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–90. https://doi.org/10.1016/s0140-6736(02)08778-0.
Shepherd ES, Goldsmith S, Doyle LW, Middleton P, Marret S, Rouse DJ, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2024;5:CD004661 https://doi.org/10.1002/14651858.CD004661.pub4.
Wolf HT, Brok J, Henriksen TB, Greisen G, Salvig JD, Pryds O, et al. MASP research group. Antenatal magnesium sulphate for the prevention of cerebral palsy in infants born preterm: a double-blind, randomised, placebo-controlled, multi-centre trial. BJOG. 2020;127:1217–25. https://doi.org/10.1111/1471-0528.16239.
Neilson JP, West HM, Dowswell T. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2014;2014:CD004352 https://doi.org/10.1002/14651858.CD004352.pub3.
Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2013;2013:CD000940 https://doi.org/10.1002/14651858.CD000940.pub3.
Flenady V, Wojcieszek AM, Papatsonis DN, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database Syst Rev. 2014;2014:CD002255. https://doi.org/10.1002/14651858.CD002255.pub2.
Ara I, Banu H. A prospective randomised trial of nifedipine versus placebo in preterm labour. Bangladesh J Obstet Gynecol. 2008;23:61–4.
Zhang X, Liu M. Clinical observations on the prevention and treatment of premature labor with nifedipine. Hua‐Hsi i Ko Ta Hsueh Hsueh Pao [J West China Univ Med Sci]. 2002;33:288–90.
Reinebrant HE, Pileggi-Castro C, Romero CL, Dos Santos RA, Kumar S, Souza JP, et al. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2015;2015:CD001992 https://doi.org/10.1002/14651858.CD001992.pub3.
Panter KR, Hannah ME, Amankwah KS, Ohlsson A, Jefferies AL, Farine D. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo‐controlled trial. Br J Obstet Gynaecol. 1999;106:467–73.
Mercer BM, Miodovnik M, Thurnau GR, Goldenberg RL, Das AF, Ramsey RD, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989–95.
Mercer B, Moretti M, Rogers R, Sibai B. Antibiotic prophylaxis in preterm premature rupture of the membranes: prospective randomized doubleblind trial of 220 patients. Am J Obstet Gynecol. 1992;166:794–802.
Günes A, Kiyak H, Yüksel S, Bolluk G, Erbiyik RM, Gedikbasi A. Predicting previable preterm premature rupture of membranes (pPPROM) before 24 weeks: maternal and fetal/neonatal risk factors for survival. J Obstet Gynaecol. 2022;42:597–606. https://doi.org/10.1080/01443615.2021.1935818.
LeMoine F, Moore RC, Chapple A, Moore FA, Sutton E. Neonatal survivability following previable PPROM after hospital readmission for intervention. AJP Rep. 2020;10:e395–e402. https://doi.org/10.1055/s-0040-1721421.
Esteves JS, de Sá RA, de Carvalho PR, Coca Velarde LG. Neonatal outcome in women with preterm premature rupture of membranes (PPROM) between 18 and 26 weeks. J Matern Fetal Neonatal Med. 2016;29:1108–12. https://doi.org/10.3109/14767058.2015.1035643.
Knupp RJ, Pederson S, Blanchard C, Szychowski J, Etikala D, Sinkey R, et al. Antibiotic timing in previable prelabor rupture of membranes less than 24 weeks of gestation. Am J Perinatol. 2022;39:671–6. https://doi.org/10.1055/s-0040-1718876.
Society for Maternal-Fetal Medicine (SMFM), Battarbee AN, Osmundson SS, McCarthy AM, Louis JM, SMFM Publications Committee. Society for Maternal-Fetal Medicine Consult Series #71: Management of previable and periviable preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2024;231:B2–B15. https://doi.org/10.1016/j.ajog.2024.07.016. Electronic address: pubs@smfm.orgEpub 2024 Jul 16.
Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N. Engl J Med. 1986;314:1665–9. https://doi.org/10.1056/NEJM198606263142603.
Boyer KM, Gadzala CA, Kelly PD, Gotoff SP. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. III. Interruption of mother-to-infant transmission. J Infect Dis. 1983;148:810–6. https://doi.org/10.1093/infdis/148.5.810.
Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020 Feb;135:e51-e72 https://doi.org/10.1097/AOG.0000000000003668. Erratum in: Obstet Gynecol. 2020 Apr;135(4):978-979. https://doi.org/10.1097/AOG.0000000000003824.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 171: Management of Preterm Labor. Obstet Gynecol. 2016 Oct;128:e155-64. https://doi.org/10.1097/AOG.0000000000001711.
Helenius K, Longford N, Lehtonen L, Modi N, Gale C. Neonatal Data Analysis Unit and the United Kingdom Neonatal Collaborative. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678 https://doi.org/10.1136/bmj.l5678.
Shlossman PA, Manley JS, Sciscione AC, Colmorgen GH. An analysis of neonatal morbidity and mortality in maternal (in utero) and neonatal transports at 24-34 weeks’ gestation. Am J Perinatol. 1997;14:449–56. https://doi.org/10.1055/s-2007-994178.
Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–8. https://doi.org/10.1542/peds.2011-2820.
Hohlagschwandtner M, Husslein P, Klebermass K, Weninger M, Nardi A, Langer M. Perinatal mortality and morbidity. Comparison between maternal transport, neonatal transport and inpatient antenatal treatment. Arch Gynecol Obstet. 2001;265:113–8. https://doi.org/10.1007/s004040100197.
Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N. Engl J Med. 2007;356:2165–75. https://doi.org/10.1056/NEJMsa065029 .
Bottoms SF, Paul RH, Iams JD, Mercer BM, Thom EA, Roberts JM, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960–6. https://doi.org/10.1016/s0002-9378(97)70386-7.
Alfirevic Z, Milan SJ, Livio S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev. 2013;2013:CD000078 https://doi.org/10.1002/14651858.CD000078.pub3.
Roeckner JT, Peterson E, Rizzo J, Flores-Torres J, Odibo AO, Duncan JR. The impact of mode of delivery on maternal and neonatal outcomes during periviable birth (22-25 weeks). Am J Perinatol. 2022;39:1269–78. https://doi.org/10.1055/a-1788-5802.
Tucker Edmonds B, McKenzie F, Macheras M, Srinivas SK, Lorch SA. Morbidity and mortality associated with mode of delivery for breech periviable deliveries. Am J Obstet Gynecol. 2015;213:70.e1–70.e12. https://doi.org/10.1016/j.ajog.2015.03.002.
Reddy UM, Zhang J, Sun L, Chen Z, Raju TN, Laughon SK. Neonatal mortality by attempted route of delivery in early preterm birth. Am J Obstet Gynecol. 2012;207:117.e1–8. https://doi.org/10.1016/j.ajog.2012.06.023.
Czarny HN, Forde B, DeFranco EA, Hall ES, Rossi RM. Association between mode of delivery and infant survival at 22 and 23 weeks of gestation. Am J Obstet Gynecol MFM. 2021;3:100340 https://doi.org/10.1016/j.ajogmf.2021.100340.
Običan SG, Small A, Smith D, Levin H, Drassinower D, Gyamfi-Bannerman C. Mode of delivery at periviability and early childhood neurodevelopment. Am J Obstet Gynecol. 2015;213:578.e1–4. https://doi.org/10.1016/j.ajog.2015.06.047.
Kawakita T, Sondheimer T, Jelin A, Reddy UM, Landy HJ, Huang CC, et al. Maternal morbidity by attempted route of delivery in periviable birth. J Matern Fetal Neonatal Med. 2021;34:1241–8. https://doi.org/10.1080/14767058.2019.1631792.
Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomized trial. BMJ. 1993;306:172–5.
McDonnell M, Henderson-Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. J Paediatr Child Health. 1997;33:308–10.
Rabe H, Wacker A, Hulskamp G, et al. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. Eur J Pediatr. 2000;159:775–7.
Ibrahim HM, Krouskop RW, Lewis DF, Dhanireddy R. Placental transfusion: umbilical cord clamping and preterm infants. J Perinatol. 2000;20:351–4.
Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23:466–72. https://doi.org/10.1038/sj.jp.7210970.
Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Australian Placental Transfusion Study Collaborative Group. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377:2445–55. https://doi.org/10.1056/NEJMoa1711281.
Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322:1877–86. https://doi.org/10.1001/jama.2019.16004.
Seidler AL, Aberoumand M, Hunter KE, Barba A, Libesman S, Williams JG, et al. iCOMP Collaborators. Deferred cord clamping, cord milking, and immediate cord clamping at preterm birth: a systematic review and individual participant data meta-analysis. Lancet. 2023;402:2209–22. https://doi.org/10.1016/S0140-6736(23)02468-6.
Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–71. https://doi.org/10.1542/peds.106.4.659.
Author information
Authors and Affiliations
Consortia
Contributions
FL contributed to data curation, and manuscript writing, review, and editing. AB, CT, CB, and KG contributed to data contribution and manuscript review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
LeMoine, F.V., Battarbee, A.N., Travers, C.P. et al. Considerations for obstetric management of births 22–25 weeks’ gestation. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02289-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-025-02289-y