Abstract
Objective
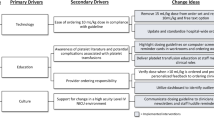
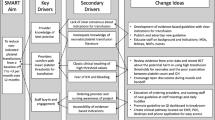
Thrombocytopenia is a common problem affecting preterm neonates. Recent studies show increased morbidity and mortality with liberal platelet transfusion thresholds. We sought to standardize thrombocytopenia management through a transfusion guideline to reduce excessive transfusions.
Study design
We developed and implemented a guideline using PDSA cycles for infants with birth weights <1000 grams. Platelet transfusions were classified as indicated or non-indicated per the guideline. Severe (grade 3 or 4) intraventricular hemorrhage and pulmonary hemorrhage rates were balancing measures.
Results
We analyzed 101 infants pre-guideline and 96 infants post-guideline. The mean monthly non-indicated transfusions significantly decreased from 13.0 to 2.0, respectively (p-value < 0.01). Incidence of severe grade IVH and pulmonary hemorrhage remained stable.
Conclusion
Rapid implementation of an evidence-based, restrictive platelet transfusion guideline significantly reduced non-indicated platelet transfusions without a concomitant increase in major bleeding events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ghadeer HAA, Aldhahi RA, Dandan FKA, Alamer MH, Almulaifi LF, Muaibid AFA, et al. The prevalence and associated risk factors for neonatal thrombocytopenia among newborns admitted to the neonatal intensive care unit. Cureus. 2024;16:e56108.
Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348–53.
Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749–55.
Roberts I. Neonatal thrombocytopenia: causes and management. Arch Dis Child—Fetal Neonatal Ed. 2003;88:359F–364.
Christensen RD, Baer VL, Henry E, Snow GL, Butler A, Sola-Visner MC. Thrombocytopenia in small-for-gestational-age infants. Pediatrics. 2015;136:e361.
Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. 2019;380:242–51.
Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124:e826–34.
Moore CM, D’Amore A, Fustolo-Gunnink S, Hudson C, Newton A, Santamaria BL, et al. Two-year outcomes following a randomised platelet transfusion trial in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2023;108:452.
Bahr TM, Snow GL, Christensen TR, Davenport P, Henry E, Tweddell SM, et al. Can red blood cell and platelet transfusions have a pathogenic role in bronchopulmonary dysplasia? J Pediatr. 2024;265:113836.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
Montgomery DC. Statistical quality control: a modern introduction. 6th ed. Hoboken, NJ. John Wiley & Sons; 2010. p. 756.
SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process–PMC [Internet]. [cited 2024 Oct 21]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4625997/
Garcia MG, Duenas E, Sola MC, Hutson AD, Theriaque D, Christensen RD. Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol. 2001;21:415–20.
Moore CM, O’Reilly D, McCallion N, Curley AE. Changes in inflammatory proteins following platelet transfusion in a neonatal population. Pediatr Res. 2023;94:1973–7.
Garraud O, Cognasse F, Tissot JD, Chavarin P, Laperche S, Morel P, et al. Improving platelet transfusion safety: biomedical and technical considerations. Blood Transfus. 2016;14:109–22.
Stolla M, Refaai MA, Heal JM, Spinelli SL, Garraud O, Phipps RP, et al. Platelet transfusion—the new immunology of an old therapy. Front Immunol. 2015;6:28.
Sola-Visner M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematology. 2012;2012:506–11.
So K, Fok T, Ng P, Wong W, Cheung K. Randomised controlled trial of colloid or crystalloid in hypotensive preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;76:F43–6.
Shalish W, Olivier F, Aly H, Sant’Anna G. Uses and misuses of albumin during resuscitation and in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2017;22:328–35.
Oca MJ, Nelson M, Donn SM. Randomized trial of normal saline versus 5% albumin for the treatment of neonatal hypotension. J Perinatol. 2003;23:473–6.
Emery EF, Greenough A, Gamsu HR. Randomised controlled trial of colloid infusions in hypotensive preterm infants. Arch Dis Child. 1992;67:1185–8.
Bignall S, Bailey PC, Bass CA, Cramb R, Rivers RPA, Wadsworth J. The cardiovascular and oncotic effects of albumin infusion in premature infants. Early Hum Dev. 1989;20:191–201.
Davenport PE, Yuen JC, Briere J, Feldman HA, Sola-Visner MC, Leeman KT. Implementation of a neonatal platelet transfusion guideline to reduce non-indicated transfusions using a quality improvement framework. J Perinatol. 2021;41:1487.
Bahr TM, Christensen TR, Henry E, Astin M, Ilstrup SJ, Ohls RK, et al. Platelet transfusions in a multi-neonatal intensive care unit health care organization before and after publication of the PlaNeT-2 clinical trial. J Pediatr. 2023;257:113388.
Chotas W, Wallman-Stokes A, Patel RM, Cooper C, Soll R. Platelet transfusion thresholds for thrombocytopenic infants. Cochrane Database Syst Rev. 2024;2024:CD015341.
Author information
Authors and Affiliations
Contributions
NL, GP and AL all contributed to study design. NL and AB contributed to collection and assembly of data. NL completed analysis and interpretation of data. LV contributed to verification of quality improvement metrics. NL, GP, LV and AL contributed to manuscript preparation, writing, editing, and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lalos, N., Brumfiel, A., Viehl, L.T. et al. Development and implementation of restrictive platelet transfusion thresholds in a neonatal intensive care unit. J Perinatol 45, 1833–1838 (2025). https://doi.org/10.1038/s41372-025-02302-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02302-4
This article is cited by
-
Using a lower platelet transfusion threshold: translating evidence into practice
Journal of Perinatology (2025)