Abstract
Objective
To describe the effects of dexmedetomidine on sedation, pain, respiratory status, and hemodynamics in neonates.
Methods
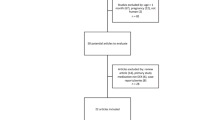
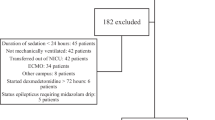
A retrospective study of 206 infants in a level IV NICU (2016–2021) receiving continuous dexmedetomidine infusion. Pain/sedation scores, BP, vasopressor and inotrope score (VIS), and concomitant sedatives/analgesics (CSA) were recorded before and every 3–4 h for 24 h.
Results
Median PMA:32 weeks. Hypotension occurred in 26%, primarily in infants <32weeks PMA, correlating with higher VIS and CSA. CSA use significantly predicted vasopressors/inotrope use.
Conclusion
Dexmedetomidine, with CSA, increases cardiovascular instability in preterm infants who have unique myocardial structure and function and therefore higher vulnerability.
Clinical Trial Registration (if any)
None (not applicable).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets presented in this article are not publicly available, as they contain de-identified data retrospectively collected from medical records, consistent with local IRB regulations. Requests regarding dataset access should be directed to the corresponding author, Marjorie M. Makoni (marjorie-mkoni@ouhsc.edu).
References
Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: a systematic review. Clin J Pain. 2015;31:355–62.
American Academy of Pediatrics Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137:e20154271.
Hall RW. Anesthesia and analgesia in the NICU. Clin Perinatol. 2012;39:239–54.
Mahmoud M, Barbi E, Mason KP. Dexmedetomidine: what’s new for pediatrics? A narrative review. J Clin Med, 2020;9::2724.
Dilek O, Yasemin G, Atci M. Preliminary experience with dexmedetomidine in neonatal anesthesia. J Anaesthesiol Clin Pharm. 2011;27:17–22.
Arcangeli A, D’Alo C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10:687–95.
Chen J, Shen N, Duan X, Guo Y. An investigation of the mechanism of dexmedetomidine in improving postoperative cognitive dysfunction from the perspectives of alleviating neuronal mitochondrial membrane oxidative stress and electrophysiological dysfunction. Exp Ther Med. 2018;15:2037–43.
Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: a meta-analysis of randomised controlled trials. Anaesthesia. 2008;63:4–14.
Mason KP, Zgleszewski SE, Dearden JL, Dumont RS, Pirich MA, Stark CD, et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006;103:57–62.
Lam F, Bhutta AT, Tobias JD, Gossett JM, Morales L, Gupta P. Hemodynamic effects of dexmedetomidine in critically ill neonates and infants with heart disease. Pediatr Cardiol. 2012;33:1069–77.
Sellas MN, Kyllonen KC, Lepak MR, Rodgriguez RJ. Dexmedetomidine for the management of postoperative pain and sedation in newborns. J Pediatr Pharm Ther. 2019;24:227–33.
Hogue CW Jr, Talke P, Stein PK, Richardson C, Domitrovich PP, et al. Autonomic nervous system responses during sedative infusions of dexmedetomidine. Anesthesiology. 2002;97:592–8.
Kim MY, Finch AM, Lumbers ER, Boyce AC, Gibson KJ, Eiby YA, et al. Expression of adrenoceptor subtypes in preterm piglet heart is different to term heart. PLoS One. 2014;9:e92167.
Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53.
Walker CA, Spinale FG. The structure and function of the cardiac myocyte: a review of fundamental concepts. J Thorac Cardiovasc Surg. 1999;118:375–82.
Stranak Z, Semberova J, Barrington K, O’Donnell C, Marlow N, Maulaers G, et al. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur J Pediatr. 2014;173:793–8.
Takemoto CK Pediatric and Neonatal Dosage Handbook, 29th ed. Hudson, OH, 2022.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Raffaeli G, Cristofori G, Befani B, De Carli A, Cavallaro G, Fumagalli M, et al. EDIN Scale implemented by gestational age for pain assessment in preterms: a prospective study. Biomed Res Int. 2017;2017:9253710.
Kerson AG, DeMaria R, Mauer E, Joyce C, Gerber LM, Greenwald BM, et al. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. J Intensive Care. 2016;4:65.
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–8.
McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the Vasoactive-Inotropic Score in pediatric sepsis. Pediatr Crit Care Med. 2017;18:750–7.
Giesinger RE, McNamara PJ. Hemodynamic instability in the critically ill neonate: an approach to cardiovascular support based on disease pathophysiology. Semin Perinatol. 2016;40:174–88.
Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34:216–21.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch Dis Child Fetal Neonatal Ed. 2004;89:F168–73.
Kharrat A, Rios DI, Weisz DE, Giesinger RE, Groves A, Yang J, et al. The relationship between blood pressure parameters and left ventricular output in neonates. J Perinatol. 2019;39:619–25.
Mullaly R, El-Khuffash AF. Haemodynamic assessment and management of hypotension in the preterm. Arch Dis Child Fetal Neonatal Ed. 2024;109:120–7.
Bishop SP, Zhang J, Ye L Cardiomyocyte proliferation from fetal- to adult- and from normal- to hypertrophy and failing hearts. Biology (Basel), 2022;11.
Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi-Harami F, et al. Contractile properties of developing human fetal cardiac muscle. J Physiol. 2016;594:437–52.
Loescher CM, Freundt JK, Unger A, Hessel AL, Kühn M, Koser F, et al. Titin governs myocardial passive stiffness with major support from microtubules and actin and the extracellular matrix. Nat Cardiovascular Res. 2023;2:991–1002.
Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62:31–9.
Aziz KB, Lavilla OC, Wynn JL, Lure AC, Gipson D, de la Cruz D. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J Perinatol. 2021;41:2337–44.
Cioccari L, Luethi N, Bailey M, Shehabi Y, Howe B, Messmer AS, et al. The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: a subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit Care. 2020;24:441.
Whalen LD, Di Gennaro JL, Irby GA, Yanay O, Zimmerman JJ. Long-term dexmedetomidine use and safety profile among critically ill children and neonates. Pediatr Crit Care Med. 2014;15:706–14.
Chrysostomou C, Schulman SR, Castellanos MH, Cofer BE, Mitra S, da Rocha MG, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164:276–82.e1-3
O’Mara K, Gal P, Wimmer J, Ransom JL, Carlos RQ, Dimaguila MAVT, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharm Ther. 2012;17:252–62.
Morton SU, Labrecque M, Moline M, Hansen A, Leeman K Reducing benzodiazepine exposure by instituting a guideline for dexmedetomidine usage in the NICU. Pediatrics, 2021;148:e2020041566.
Dersch-Mills DA, Banasch JL, Yusuf K, Howlett A. Dexmedetomidine use in a tertiary care NICU: a descriptive study. Ann Pharmacother. 2019;53:464–70.
Funding
The author(s) declare that financial support was received for this article’s research, authorship, and/or publication. Research reported in this publication was supported by the National Institute on Minority Health & Health Disparities of the National Institutes of Health under award Number R25MD011564. This content is solely the responsibility of the Authors and does not represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
MMM conceptualized and designed the study, coordinated and supervised data collection, assisted in drafting the initial manuscript, and revised the manuscript. ISS assisted in writing the study protocol, obtaining IRB approval, collecting data, and drafting the initial manuscript and early revisions of the manuscript. ARB assisted in conceptualizing and organizing the study, conducted a comprehensive review of the manuscript, identifying areas for improvement, restructuring the paper, enhancing its flow, coherence, and overall organization. JLM provided guidance on pharmaceutical aspects of the study and assisted with writing, critically reviewing and revising the manuscript. ES guided the study’s conceptualization and design, provided a critical review, and assisted with revising the manuscript. LD advised on statistical analyses, oversaw the database and secure data storage, analyzed the data, and assisted with writing and revising the manuscript. WHB advised on statistical analyses, analyzed the data, created the graphs, and assisted with writing the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Statement
The studies involving humans were approved by the University of Oklahoma Health Sciences Center IRB. The study was conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of the study consent was waived (IRB #13410).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article summary: This study describes the use and effects of dexmedetomidine across all postmenstrual ages in a neonatal intensive care unit.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makoni, M.M., Sierra-Strum, I., Bischoff, A.R. et al. Dexmedetomidine’s effect on neonatal sedation, pain, respiratory status and cardiovascular system. J Perinatol 45, 1686–1692 (2025). https://doi.org/10.1038/s41372-025-02339-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02339-5