Abstract
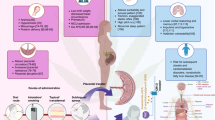
The global prevalence of cannabis use during pregnancy is increasing, driven by perceived therapeutic benefits and greater societal acceptance. Concurrently, the psychoactive potency of cannabis products has risen significantly, due to increase in concentrations of Tetrahydrocannabinol (THC) from 5% to 30%. THC crosses the placenta, disrupts the endocannabinoid system critical for neurodevelopment, and accumulates in fetal tissues. THC is transferred into breast milk, with breastfed infants receiving ~2.5% of the maternal dose, raising concerns regarding neurodevelopmental consequences. An increasing number of studies and metanalysis have demonstrated association of prenatal cannabis exposure with low birth weight, preterm birth, neonatal intensive care unit admission, and reduced Apgar scores. Longitudinal studies show brain alterations in offspring, affecting memory, attention, and executive function. The inability to conduct randomized controlled trials due to ethical constraints necessitates reliance on observational studies, underscoring the need for rigorous longitudinal research to delineate causality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hartsel JA, Eades J, Hickory B, Makriyannis A. Cannabis sativa and Hemp. Nutraceuticals. 2016;735–54. https://linkinghub.elsevier.com/retrieve/pii/B978012802147700053X.
Blebea NM, Pricopie AI, Vlad RA, Hancu G. Phytocannabinoids: exploring pharmacological profiles and their impact on therapeutical use. Int J Mol Sci. 2024;25:4204.
Health Canada. About cannabis. Government of Canada. 2025. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/about.html.
ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79:613–9.
Lo JO, Hedges JC, Metz TD. Cannabis use and perinatal health research. JAMA. 2023;330:913–4.
United Nations Office on Drugs and Crime. Cannabis and hallucinogens. In: World Drug Report 2019. UN; 2019. pp. 1–73. https://www.un-ilibrary.org/drugs-crime-and-terrorism/world-drug-report-2019_5b5a0f55-en.
Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322:167–9.
Health Canada. Canadian Cannabis Survey 2023: Summary. Canada.ca. 2023. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2023-summary.html.
Luke S, Hutcheon J, Kendall T. Cannabis use in pregnancy in British Columbia and selected birth outcomes. J Obstet Gynaecol Can. 2019;41:1311–7.
Luke S, Hobbs AJ, Smith M, Riddell C, Murphy P, Agborsangaya C, et al. Cannabis use in pregnancy and maternal and infant outcomes: a Canadian cross-jurisdictional population-based cohort study. PLoS One. 2022;17:e0276824.
Bayrampour H, Asim A. Cannabis use during the pre-conception period and pregnancy after legalization. J Obstet Gynaecol Can. 2021;43:740–5.
Myran DT, Roberts R, Pugliese M, Corsi D, Walker M, El-Chaâr D, et al. Acute care related to cannabis use during pregnancy after the legalization of nonmedical cannabis in Ontario. CMAJ. 2023;195:E699–708.
ACOG. Committee opinion No. 722: marijuana use during pregnancy and lactation. Obstet Gynecol. 2017;130:e205–e209.
Center for Behavioral Health Statistics and Quality. 2019 National Survey on Drug Use and Health: Detailed Tables. Substance abuse and mental health services administration. 2020. https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables. Accessed February 19, 2023.
Young-Wolff KC, Ray GT, Alexeeff SE, Adams SR, Does MB, Ansley D, et al. Rates of prenatal cannabis use among pregnant women before and during the COVID-19 pandemic. JAMA. 2021;326:1745–7.
Fergusson DM, Horwood LJ, Northstone K. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109:21–7.
Barbosa-Leiker C, Burduli E, Smith CL, Brooks O, Orr M, Gartstein M. Daily cannabis use during pregnancy and postpartum in a state with legalized recreational cannabis. J Addict Med. 2020;14:467–74.
Argueta DA, Aich A, Muqolli F, Cherukury H, Sagi V, DiPatrizio NV, et al. Considerations for cannabis use to treat pain in sickle cell disease. J Clin Med. 2020;9:3902.
Young-Wolff KC, Gali K, Sarovar V, Rutledge GW, Prochaska JJ. Women’s questions about perinatal cannabis use and health care providers’ responses. J Womens Health. 2020;29:919–26.
Mark K, Gryczynski J, Axenfeld E, Schwartz RP, Terplan M. Pregnant women’s current and intended cannabis use in relation to their views toward legalization and knowledge of potential harm. J Addict Med. 2017;11:211–6.
Chang JC, Tarr JA, Holland CL, De Genna NM, Richardson GA, Rodriguez KL, et al. Beliefs and attitudes regarding prenatal marijuana use: perspectives of pregnant women who report use. Drug Alcohol Depend. 2019;196:14–20.
Postonogova T, Xu C, Moore A. Marijuana during labour: a survey of maternal opinions. J Obstet Gynaecol Can. 2020;42:774–8.
Chabarria KC, Racusin DA, Antony KM, Kahr M, Suter MA, Mastrobattista JM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1–7.
Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25.
Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharm. 2005;168:299–325.
Fernández-Ruiz J, Hernández M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–91.
Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–59.
Taylor AH, Amoako AA, Bambang K, Karasu T, Gebeh A, Lam PM, et al. Endocannabinoids and pregnancy. Clin Chim Acta. 2010;411:921–30.
Paria BC, Dey SK. Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids. 2000;108:211–20.
Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276:20523–8.
Sun X, Dey SK. Aspects of endocannabinoid signaling in periimplantation biology. Mol Cell Endocrinol. 2008;286:S3–11.
Galve-Roperh I, Palazuelos J, Aguado T, Guzmán M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2009;259:371–82.
Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharm Sci. 2007;28:83–92.
Anavi‐Goffer S, Mulder J. The polarised life of the endocannabinoid system in CNS development. ChemBioChem. 2009;10:1591–8.
De Salas-Quiroga A, Díaz-Alonso J, García-Rincón D, Remmers F, Vega D, Gómez-Cañas M, et al. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB 1 receptors on developing cortical neurons. Proc Natl Acad Sci USA. 2015;112:13693–8.
Zurolo E, Iyer AM, Spliet WG, Van Rijen PC, Troost D, Gorter JA, et al. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience. 2010;170:28–41.
Biegon A, Kerman IA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. NeuroImage. 2001;14:1463–8.
Bara A, Ferland JN, Rompala G, Szutorisz H, Hurd YL. Cannabis and synaptic reprogramming of the developing brain. Nat Rev Neurosci. 2021;22:423–38.
Natale BV, Gustin KN, Lee K, Holloway AC, Laviolette SR, Natale DRC, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci Rep. 2020;10:544.
Maroon J, Bost J. Review of the neurological benefits of phytocannabinoids. Surg Neurol Int. 2018;9:91.
Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des. 2014;20:4112–8.
Narendran N, Yusuf K. Marijuana Use during Pregnancy and Lactation and Long-term Outcomes. NeoReviews. 2021;22:e521–30.
Hindley G, Beck K, Borgan F, Ginestet E, McCutcheon R, Kleinloog D, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7:344–53.
Bailey JR, Cunny HC, Paule MG, Slikker W Jr. Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol Appl Pharm. 1987;90:315–21.
Behnke M, Smith VC. Committee on Substance Abuse; Committee on Fetus and Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009–24.
Abrams RM, Cook CE, Davis KH, Niederreither K, Jaeger MJ, Szeto HH. Plasma delta-9-tetrahydrocannabinol in pregnant sheep and fetus after inhalation of smoke from a marijuana cigarette. Alcohol Drug Res. 1985;6:361–9.
Lee MJ. Marihuana and tobacco use in pregnancy. Obstet Gynecol Clin North Am. 1998;25:65–83.
Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701.
Schlienz NJ, Spindle TR, Cone EJ, Herrmann ES, Bigelow GE, Mitchell JM, et al. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend. 2020;211:107969.
Klausner HA, Dingell JV. The metabolism and excretion of delta 9-tetrahydrocannabinol in the rat. Life Sci I. 1971;10:49–59.
Kreuz DS, Axelrod J. Delta-9-tetrahydrocannabinol: localization in body fat. Science. 1973;179:391–3.
Ho BT, Fritchie GE, Kralik PM, Englert LF, McIsaac WM, Idänpään-Heikkiläj, et al. Distribution of tritiated-1 delta 9 tetrahydrocannabinol in rat tissues after inhalation. J Pharm Pharm. 1970;22:538–9.
Westin AA, Huestis MA, Aarstad K, Spigset O. Urinary excretion of 11-Nor-9-carboxy-Δ9-tetrahydrocannabinol in a pregnant woman following heavy, chronic cannabis use. J Anal Toxicol. 2009;33:610–4.
Monfort A, Ferreira E, Leclair G, Lodygensky GA. Pharmacokinetics of cannabis and its derivatives in animals and humans during pregnancy and breastfeeding. Front Pharm. 2022;13:919630.
Williams LJ, Correa A, Rasmussen S. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res A Clin Mol Teratol. 2004;70:59–64.
Van Gelder MMHJ, Donders ART, Devine O, Roeleveld N, Reefhuis J. National birth defects prevention study. using bayesian models to assess the effects of under‐reporting of cannabis use on the association with birth defects, national birth defects prevention study, 1997–2005. Paediatr Perinat Epid. 2014;28:424–33.
Bourque DK, Meng L, Dougan S, Momoli F, Riddell C, Walker M, et al. Gastroschisis in Ontario, Canada: 2012-2018. Birth Defects Res. 2021;113:1044–51.
Delker E, Hayes S, Kelly AE, Jones KL, Chambers C, Bandoli G. Prenatal exposure to cannabis and risk of major structural birth defects: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:269–83.
Gunn JK, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6:e009986.
Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:713–23.
Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145–52.
Shi Y, Zhu B, Liang D. The associations between prenatal cannabis use disorder and neonatal outcomes. Addiction. 2021;116:3069–79.
Nguyen VH, Harley KG. Prenatal cannabis use and infant birth outcomes in the pregnancy risk assessment monitoring system. J Pediatr. 2022;240:87–93.
Marchand G, Masoud AT, Govindan M, Ware K, King A, Ruther S, et al. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2145653.
Sorkhou M, Singla DR, Castle DJ, George TP. Birth, cognitive and behavioral effects of intrauterine cannabis exposure in infants and children: a systematic review and meta-analysis. Addiction. 2024;119:411–37.
Lo JO, Shaw B, Robalino S, Ayers CK, Durbin S, Rushkin MC, et al. Cannabis use in pregnancy and neonatal outcomes: a systematic review and meta-analysis. Cannabis Cannabinoid Res. 2024;9:470–85.
Avalos LA, Adams SR, Alexeeff SE, Oberman NR, Does MB, Ansley D, et al. Neonatal outcomes associated with in utero cannabis exposure: a population-based retrospective cohort study. Am J Obstet Gynecol. 2024;231:132.e1–132.e13.
Metz TD, Allshouse AA, McMillin GA, Greene T, Chung JH, Grobman WA, et al. Cannabis exposure and adverse pregnancy outcomes related to placental function. JAMA. 2023;330:2191–9.
Duko B, Dachew BA, Pereira G, Alati R. The effect of prenatal cannabis exposure on offspring preterm birth: a cumulative meta-analysis. Addiction. 2023;118:607–19.
Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347–51.
Dewey WL. Cannabinoid pharmacology. Pharm Rev. 1986;38:151–78.
Dotters-Katz SK, Smid MC, Manuck TA, Metz TD. Risk of neonatal and childhood morbidity among preterm infants exposed to marijuana. J Matern Fetal Neonatal Med. 2017;30:2933–9.
Prewitt KC, Hayer S, Garg B, Benson AE, Hedges MA, Caughey AB, et al. Impact of prenatal cannabis use disorder on perinatal outcomes. J Addict Med. 2023;17:e192–198.
Metz TD, Allshouse AA, Hogue CJ, Goldenberg RL, Dudley DJ, Varner MW, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. 2017;217:478.e1–478.e8.
Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–23.
Cabral GA, Marciano-Cabral F. Cannabinoid-mediated exacerbation of brain infection by opportunistic amebae. J Neuroimmunol. 2004;147:127–30.
Cabral GA, Vásquez R. Effects of marijuana on macrophage function. Adv Exp Med Biol. 1991;288:93–105.
Klein TW, Newton C, Friedman H. Resistance to Legionella pneumophila suppressed by the marijuana component, tetrahydrocannabinol. J Infect Dis. 1994;169:1177–9.
Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. 1990;11:49–58.
Fried PA, O’Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr. 1992;13:383–91.
Sharapova SR, Phillips E, Sirocco K, Kaminski JW, Leeb RT, Rolle I. Effects of prenatal marijuana exposure on neuropsychological outcomes in children aged 1-11 years: a systematic review. Paediatr Perinat Epidemiol. 2018;32:512–32.
Smith AM, Mioduszewski O, Hatchard T, Byron-Alhassan A, Fall C, Fried PA. Prenatal marijuana exposure impacts executive functioning into young adulthood: an fMRI study. Neurotoxicol Teratol. 2016;58:53–59.
McLemore GL, Richardson KA. Data from three prospective longitudinal human cohorts of prenatal marijuana exposure and offspring outcomes from the fetal period through young adulthood. Data Brief. 2016;9:753–7.
El Marroun H, Tiemeier H, Franken IH, Jaddoe VW, van der Lugt A, Verhulst FC, et al. Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biol Psychiatry. 2016;79:971–9.
Kooijman MN, Kruithof CJ, Van Duijn CM, Duijts L, Franco OH, Van IJzendoorn MH, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64.
Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, et al. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD Study. JAMA Psychiatry. 2021;78:64–76.
Talavera-Barber MM, Morehead E, Ziegler K, Hockett C, Elliott AJ. Prenatal cannabinoid exposure and early language development. Front Pediatr. 2023;11:1290707.
Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicol Teratol. 2016;58:5–14.
Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307:819–20.
Djulus J, Moretti M, Koren G. Marijuana use and breastfeeding. Can Fam Phys. 2005;51:349–50.
Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–804.
Baker T, Datta P, Rewers-Felkins K, Thompson H, Kallem RR, Hale TW. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783–8.
Astley SJ, Little RE. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol. 1990;12:161–8.
Shieh C, Kravitz M. Severity of drug use, initiation of prenatal care, and maternal-fetal attachment in pregnant marijuana and cocaine/heroin users. J Obstet Gynecol Neonatal Nurs. 2006;35:499–508.
Sachs HC. Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132:e796–809.
Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135–41.
Society of Obstetricians and Gynaecologists of Canada. (2017a). SOGC Position Statement: marijuana use during Pregnancy. 2017. Retrieved from https://sogc.org/files/letSOGCstatementCannabisUse.pdf.
Author information
Authors and Affiliations
Contributions
BT contributed to writing of the original draft, manuscript preparation, literature review, reviewing, and editing. KY contributed to conceptualization, provided the intellectual framework for the manuscript, supervised manuscript preparation, contributed to the literature review, and extensively reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thayyil, B., Yusuf, K. Evidence on the effect of in-utero cannabis exposure in neonates. J Perinatol 45, 1503–1512 (2025). https://doi.org/10.1038/s41372-025-02383-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02383-1