Abstract
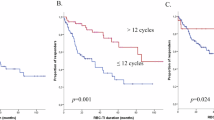
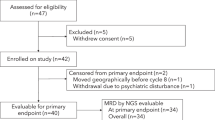
The delayed intensification (DI) enhanced outcome for patients with acute lymphoblastic leukemia (ALL) treated on BFM 76/79 and CCG 105 after a prednisone-based induction. Childrens Oncology Group protocols P9904/9905 evaluated DI via a post-induction randomization for eligible National Cancer Institute (NCI) standard (SR) and high-risk (HR) patients. A second randomization compared intravenous methotrexate (IV MTX) as a 24- (1 g/m2) vs. 4-h (2 g/m2) infusion. NCI SR patients received a dexamethasone-based three-drug and NCI HR/CNS 3 SR patients a prednisone-based four-drug induction. End induction MRD (minimal residual disease) was obtained but did not impact treatment. DI improved the 10-year continuous complete remission (CCR) rate; 75.5 ± 2.5% vs. 81.8 ± 2.2% p = 0.002, whereas MTX administration did not; 4-h 80.8 ± 1.9%; 24-h 81.4 ± 1.9% (p = 0.7780). Overall survival (OS) at 10 years did not differ with DI: 91.4 ± 1.6% vs. 90.9 ± 1.7% (p = 0.25) without but was higher with the 24-h MTX infusion; 4-h 91.1 ± 1.4%; 24-h 93.9 ± 1.2% (p = 0.0209). MRD predicted outcome; 10-year CCR 87.7 ± 2.2 and 82.1 ± 2.5% when MRD was <0.01% with/without DI (p = 0.007) and 54.3 ± 8% and 44 ± 8% for patients with MRD ≥ 0.01% with/without DI (p = 0.11). DI improved CCR for patients with B-ALL with and without end induction MRD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pui C, Robison LL, Look AT. Acute lymphoblastic leukemia. Lancet. 2008;371:1030–43.
O’Leary M, Krailo M, Anderson JR, Reaman GH. Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol. 2008;35:484–93.
Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Semin Hematol. 2009;46:52–63.
Farber S, Diamond LK, Mercer RD. Temporary remissions in acute leukemia in children produced by folic acid antagonist 4-aminopteroylglutamic acid (aminopterin). New Engl J Med. 1948;238:787–93.
Gustafsson G, Schmiegelow K, Forestier E, Clausen N, Glomstein A, Jonmundsson G, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO). Leukemia. 2000;14:2267–75.
Arico M, Baruchel A, Bertrand Y, Biondi A, Conter V, Eden T, et al. The seventh international childhood acute lymphoblastic leukemia workshop report: Palermo, Italy, January 29–30, 2005. Leukemia. 2005;19:1145–52.
Pui C. Acute lymphoblastic leukemia in children. Curr Opin Oncol. 2000;12:3–12.
Childhood ALL Collaborative Group. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Lancet. 1996;347:1783–8.
Henze G, Langermann HJ, Bramswig J, Breu H, Gadner H, Schellong G, et al. The BFM 76/79 acute lymphoblastic leukemia therapy study (author’s transl). Klin Padiatr. 1981;193:145–54.
Camitta B, Mahoney D, Leventhal B, Lauer SJ, Shuster JJ, Adair S, et al. Intensive intravenous methotrexate and mercaptopurine treatment of higher-risk non-T, non-B acute lymphocytic leukemia: A Pediatric Oncology Group study. J Clin Oncol. 1994;12:1383–9.
Land VJ, Shuster JJ, Crist WM, Ravindranath Y, Harris MB, Krance RA, et al. Comparison of two schedules of intermediate-dose methotrexate and cytarabine consolidation therapy for childhood B-precursor cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1994;12:1939–45.
Borowitz MJ, Pullen DJ, Shuster JJ, Viswanatha D, Montgomery K, Willman CL, et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children’s Oncology Group study. Leukemia. 2003;17:1566–72.
Shuster J. Identification of newly diagnosed children with acute lymphocytic leukemia at high risk for relapse. Cancer Res Ther Control. 1999;9:101–6.
Bowman WP, Larsen EL, Devidas M, Linda SB, Blach L, Carroll AJ, et al. Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukemia: results of Children’s Oncology Group trial P9906. Pediatr Blood Cancer. 2011;57:569–77.
Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children’s oncology group. Leukemia. 2010;24:355–70.
Pui C-HSJ, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of total therapy study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–6.
Harris MB, Shuster JJ, Carroll A, Look AT, Borowitz MJ, Crist WM, et al. Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood. 1992;79:3316–24.
Chauvenet AR, Martin PL, Devidas M, Linda SB, Bell BA, Kurtzberg J, et al. Antimetabolite therapy for lesser-risk B-lineage acute lymphoblastic leukemia of childhood: a report from Children’s Oncology Group Study P9201. Blood. 2007;110:1105–11.
Harris MB, Shuster JJ, Pullen J, Borowitz MJ, Carroll AJ, Behm FG, et al. Treatment of children with early pre-B and pre-B acute lymphocytic leukemia with antimetabolite-based intensification regimens: a Pediatric Oncology Group Study. Leukemia. 2000;14:1570–6.
Peto RPM, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39.
Kaplan ELMP. Non-parametric estimation for incomplete observations. Am Stat Assoc. 1958;53:457–81.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220.
Kelly KP, Wells DK, Chen L, Reeves E, Mass E, Camitta B, et al. Caregiving demands and well-being in parents of children treated with outpatient or inpatient methotrexate infusion: a report from the children’s oncology group. J Pediatr Hematol Oncol. 2014;36:495–500.
Goldie JH, Coldman AJ. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984;44:3643–53.
Norton LSR. Tumor size, sensitivity to therapy and design of treatment schedules. Cancer Treat Rep. 1977;61:1307–17.
Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–50.
Lawson SEHF, Richards S, et al. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report of the Medical Research Council UKALLR1 study. Brit J Haematol. 2000;108:531–43.
Oskarsson T, Soderhall S, Arvidson J, Forestier E, Montgomery S, Bottai M, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica. 2016;101:68–76.
Clarke M, Gaynon P, Hann I, Harrison G, Masera G, Peto R, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol. 2003;21:1798–809.
Abromowitch M, Ochs J, Pui CH, Kalwinsky D, Rivera GK, Fairclough D, et al. High-dose methotrexate improves clinical outcome in children with acute lymphoblastic leukemia: St. Jude Total Therapy Study X. Med Pediatr Oncol. 1988;16:297–303.
Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:2222–30.
Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–8.
Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer. 2000;88:1964–9.
Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–17.
Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108:1165–73.
Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Moricke A, Locatelli F, et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an International Randomized Trial (AIEOP-BFM ALL 2000). J Clin Oncol. 2018;36:244–53.
Borowitz MJDM, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblatic leukemia and its relationship to other prognostic factors. A Children’s Oncology Group Study. Blood. 2008;111:5477–85.
Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–34.
Harvey RC, Mullighan CG, Wang X, Dobbin KK, Davidson GS, Bedrick EJ, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116:4874–84.
Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371:1005–15.
Loh ML, Zhang J, Harvey RC, Roberts K, Payne-Turner D, Kang H, et al. Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group TARGET Project. Blood. 2013;121:485–8.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Funding
U10 CA98543, U10 CA98413, U10 CA180886, and U10 CA180899 from the National Institutes of Health, and by St. Baldrick’s Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Hunger received consulting fees from Novartis and honoraria from Jazz Pharmaceuticals. He owns common stock in Amgen and Merck; Dr. Borowitz received honoraria from Shire Pharmaceuticals, Jazz Pharmaceuticals, and Amgen. Drs. Winick, Martin, Devidas, Shuster, Bowman, Larsen, Pullen, Carroll, Willman, Carroll, and Camitta declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Winick, N., Martin, P.L., Devidas, M. et al. Randomized assessment of delayed intensification and two methods for parenteral methotrexate delivery in childhood B-ALL: Children’s Oncology Group Studies P9904 and P9905. Leukemia 34, 1006–1016 (2020). https://doi.org/10.1038/s41375-019-0642-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-019-0642-2
This article is cited by
-
Determinants of survival after first relapse of acute lymphoblastic leukemia: a Children’s Oncology Group study
Leukemia (2024)
-
Outstanding outcomes with two low intensity regimens in children with low-risk B-ALL: a report from COG AALL0932
Leukemia (2023)
-
Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India
Trials (2022)
-
Emerging molecular subtypes and therapeutic targets in B-cell precursor acute lymphoblastic leukemia
Frontiers of Medicine (2021)