Abstract
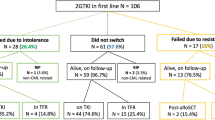
In chronic-phase chronic myeloid leukaemia (CP-CML), residual BCR-ABL1+ leukaemia stem cells are responsible for disease persistence despite TKI. Based on in vitro data, CHOICES (CHlorOquine and Imatinib Combination to Eliminate Stem cells) was an international, randomised phase II trial designed to study the safety and efficacy of imatinib (IM) and hydroxychloroquine (HCQ) compared with IM alone in CP-CML patients in major cytogenetic remission with residual disease detectable by qPCR. Sixty-two patients were randomly assigned to either arm. Treatment ‘successes’ was the primary end point, defined as ≥0.5 log reduction in 12-month qPCR level from trial entry. Selected secondary study end points were 24-month treatment ‘successes’, molecular response and progression at 12 and 24 months, comparison of IM levels, and achievement of blood HCQ levels >2000 ng/ml. At 12 months, there was no difference in ‘success’ rate (p = 0.58); MMR was achieved in 80% (IM) vs 92% (IM/HCQ) (p = 0.21). At 24 months, the ‘success’ rate was 20.8% higher with IM/HCQ (p = 0.059). No patients progressed. Seventeen serious adverse events, including four serious adverse reactions, were reported; diarrhoea occurred more frequently with combination. IM/HCQ is tolerable in CP-CML, with modest improvement in qPCR levels at 12 and 24 months, suggesting autophagy inhibition maybe of clinical value in CP-CML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3.
Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36:93–99.
Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000 ;19:2523–31.
Kuntz EM, Baquero P, Michie AM, Dunn K, Tardito S, Holyoake TL, et al. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat Med. 2017;23:1234–40.
Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–7.
Deininger M. Stem cell persistence in chronic myeloid leukemia. Leuk Suppl. 2012;1:S46–48.
Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25.
Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–10.
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Investig. 2011;121:396–409.
Kinstrie R, Karamitros D, Goardon N, Morrison H, Hamblin M, Robinson L, et al. Heterogeneous leukemia stem cells in myeloid blast phase chronic myeloid leukemia. Blood Adv. 2016;1:160–9.
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35.
Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57.
Clark RE, Polydoros F, Apperley JF, Milojkovic D, Rothwell K, Pocock C, et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 2019;6:e375–83.
Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64.
Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Investig. 2009;119:1109–23.
Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–30.
Chude CI, Amaravadi RK. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 2017;18:E1279.
Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European leukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–44.
Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90.
Lee L. NADA: Nondetects and Data Analysis for Environmental Data. 2017. https://CRAN.R-project.org/package=NADA.
R Foundation for Statistical Computing. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/.
Benjamini Y, Hochberg M. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
Baquero P, Dawson A, Mukhopadhyay A, Kuntz EM, Mitchell R, Olivares O, et al. Targeting quiescent leukemic stem cells using second generation autophagy inhibitors. Leukemia. 2019;33:981–94.
Kantarjian H, Cortes J. Considerations in the management of patients with Philadelphia chromosome-positive chronic myeloid leukemia receiving tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:1512–6.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl J Med. 2013;369:1783–96.
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–42.
Hughes TP, Lipton JH, Spector N, Cervantes F, Pasquini R, Clementino NC, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124:729–36.
Chomel JC, Bonnet ML, Sorel N, Sloma I, Bennaceur-Griscelli A, Rea D, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget. 2016;7:35293–301.
Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood. 2017;129:1595–606.
Sheng Z, Ma L, Sun JE, Zhu LJ, Green MR. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118:2840–8.
Colecchia D, Rossi M, Sasdelli F, Sanzone S, Strambi A, Chiariello M. MAPK15 mediates BCR-ABL1-induced autophagy and regulates oncogene-dependent cell proliferation and tumor formation. Autophagy. 2015;11:1790–802.
Altman BJ, Jacobs SR, Mason EF, Michalek RD, MacIntyre AN, Coloff JL, et al. Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis. Oncogene. 2011;30:1855–67.
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80.
Press RD, Galderisi C, Yang R, Rempfer C, Willis SG, Mauro MJ, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13:6136–43.
Gallipoli P, Stobo J, Heaney N, Nicolini FE, Clark R, Wilson G, et al. Safety and efficacy of pulsed imatinib with or without G-CSF versus continuous imatinib in chronic phase chronic myeloid leukaemia patients at 5 years follow-up. Br J Haematol. 2013;163:674–6.
Clark RE, Polydoros F, Apperley JF, Milojkovic D, Pocock C, Smith G, et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017;4:e310–16.
Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305.
Haas NB, Appleman LJ, Stein M, Redlinger M, Wilks M, Xu X, et al. Autophagy inhibition to augment mTOR inhibition: a phase I/II trial of everolimus and hydroxychloroquine in patients with previously treated renal cell carcinoma. Clin Cancer Res. 2019;25:2080–87.
Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1369–79.
Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1391–402.
Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, et al. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10:1380–90.
Chi KH, Ko HL, Yang KL, Lee CY, Chi MS, Kao SJ. Addition of rapamycin and hydroxychloroquine to metronomic chemotherapy as a second line treatment results in high salvage rates for refractory metastatic solid tumors: a pilot safety and effectiveness analysis in a small patient cohort. Oncotarget. 2015;6:16735–45.
Schapink L, van den Ende CHM, Gevers L, van Ede AE, den Broeder AA. The effects of methotrexate and hydroxychloroquine combination therapy vs methotrexate monotherapy in early rheumatoid arthritis patients. Rheumatology. 2019;58:131–4.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl J Med. 2003;348:994–1004.
Glivec 400 mg film-coated tablets. https://www.medicines.org.uk/emc/product/5566/smpc.
Gewirtz DA. The challenge of developing autophagy inhibition as a therapeutic strategy. Cancer Res. 2016;76:5610–4.
Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist. 2014;19:637–8.
Boone BA, Zeh HJ 3rd, Bahary N. Autophagy inhibition in pancreatic adenocarcinoma. Clin Colorectal Cancer. 2018;17:25–31.
White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–10.
Mitchell R, Hopcroft LEM, Baquero P, Allan EK, Hewit K, James D, et al. Targeting BCR-ABL-Independent TKI resistance in chronic myeloid leukemia by mTOR and autophagy inhibition. J Natl Cancer Inst. 2018;110:467–78.
McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci USA. 2012;109:8253–8.
Acknowledgements
This manuscript is dedicated to TLH (1963–2017) who was instrumental in its concept, development and delivery. The study was funded by the Medical Research Council, grant number G0900882. This study was supported by the Glasgow Experimental Cancer Medicine Centre, which is funded by Cancer Research UK and the Chief Scientist’s Office, Scotland. Cell sorting facilities were funded by the Kay Kendall Leukaemia Fund (KKL501) and the Howat Foundation. We thank the site coordinators of the study and the participants. We thank Drs David Irvine and Susan Rhodes (Beatson West of Scotland Cancer Centre, Glasgow) for consenting and processing patient samples, and Drs Bruno Calabretta (Philadelphia University) and Paolo Salomoni (DZNE, German Centre for Neurogenerative Diseases) for useful discussions. Processing of samples was performed by Dr Alan Hair and Dr Heather Jorgenson (Paul O’Gorman Leukaemia Research Centre, University of Glasgow). FACS was performed by Miss Jennifer Cassels (Paul O’Gorman Leukaemia Research Centre, University of Glasgow). We thank Kim Appleton and Chantevy Pou in the development and contribution to the HCQ PK studies (University of Glasgow). We also thank Alison Holcroft (University of Liverpool) for carrying out the plasma imatinib levels. FEN acknowledges the work, the constant administrative help and data capture for French patients of Mrs Madeleine Etienne, CRA, haematology department, Centre Hospitalier Lyon Sud, Pierre Bénite, France and of Ms Clémence Van Boxsom, CRA, Délégation à la recherche Clinique of the Hospices Civils de Lyon, Lyon. This manuscript is dedicated to TLH, who tragically passed away on 30th August 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ALL: honoraria (Kite a Gilead Company), speakers bureau (Kite a Gilead Company) and consulting or advisory role (Jazz Pharmaceuticals). JB: honoraria (Novartis, Pfizer) and speakers bureau (Novartis, Pfizer, Jazz Pharmaceuticals, Alexion). GS: research funding (Novartis, Pfizer, Ariad). SK: honoraria (Novartis, BMS, Pfizer, Incyte, Roche, AOP Pharma, Janssen, Bayer) and consulting or advisory role (Pfizer, Incyte, Novartis, AOP Pharma, BMS, CTI, Roche, Bayer). SK: research funding (Novartis, BMS, Janssen). THB: consulting or advisory role (Novartis, Pfizer, Janssen, Merck, Takeda) and research funding (Novartis, Pfizer). PS: honoraria (BMS, Novartis, Alexion, MerckSerono, Pfizer, MSD, Roche, Gilead) and consulting or advisory role (BMS, Novartis, Merck Serono, Alexion, Pfizer). PG: honoraria (BMS). FT: consulting or advisory role (bionomics) and research funding (Roche, Lilly, AstraZeneca). REC: honoraria (Novartis, Pfizer, BMS), consulting or advisory role (Novartis, Pfizer, Jazz Pharmaceuticals, Abbvie) and research funding (Novarits, BMS). DM: consultancy and honoraria (ARIAD, Bristol-Myers Squibb, Novartis, Pfizer, Incyte) and speakers bureau (Incyte). FEN: consulting or advisory role (Incyte, Sun Pharma Ltd) and speakers bureau (Incyte, BMS, Novartis). TLH (sadly passed away): research funding (Novartis, BMS), advisory board member (Novartis, Incyte), and honoraria (BMS, Novartis, Incyte). MC: research funding (Novartis, BMS, Cyclacel, Incyte), advisory board member (BMS, Novartis, Incyte, Pfizer), and honoraria (Astellas, BMS, Novartis, Incyte, Pfizer, Takeda, Celgene). The other authors have no competing financial interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Horne, G.A., Stobo, J., Kelly, C. et al. A randomised phase II trial of hydroxychloroquine and imatinib versus imatinib alone for patients with chronic myeloid leukaemia in major cytogenetic response with residual disease. Leukemia 34, 1775–1786 (2020). https://doi.org/10.1038/s41375-019-0700-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-019-0700-9
This article is cited by
-
Targeting autophagy in platinum-sensitive relapsed ovarian cancer: randomized phase II trial of hydroxychloroquine with chemotherapy with biomarker correlation
Discover Oncology (2025)
-
Potential therapeutic targets in chronic myeloid leukemia
Medical Oncology (2025)
-
Safety of Hydroxychloroquine: What a Dermatologist Should Know
American Journal of Clinical Dermatology (2025)
-
Ubiquitination regulates autophagy in cancer: simple modifications, promising targets
Journal of Translational Medicine (2024)
-
Analysis of autophagy in DLBCL reveals subtype-specific differences and the preferential targeting of ULK1 inhibition in GCB-DLBCL provides a rationale as a new therapeutic approach
Leukemia (2024)