Abstract
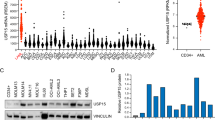
Resistance of acute myeloid leukemia (AML) to therapeutic agents is frequent. Consequently, the mechanisms leading to this resistance must be understood and addressed. In this paper, we demonstrate that inhibition of deubiquitinylase USP7 significantly reduces cell proliferation in vitro and in vivo, blocks DNA replication progression and increases cell death in AML. Transcriptomic dataset analyses reveal that a USP7 gene signature is highly enriched in cells from AML patients at relapse, as well as in residual blasts from patient-derived xenograft (PDX) models treated with clinically relevant doses of cytarabine, which indicates a relationship between USP7 expression and resistance to therapy. Accordingly, single-cell analysis of AML patient samples at relapse versus at diagnosis showed that a gene signature of the pre-existing subpopulation responsible for relapse is enriched in transcriptomes of patients with a high USP7 level. Furthermore, we found that USP7 interacts and modulates CHK1 protein levels and functions in AML. Finally, we demonstrated that USP7 inhibition acts in synergy with cytarabine to kill AML cell lines and primary cells of patients with high USP7 levels. Altogether, these data demonstrate that USP7 is both a marker of resistance to chemotherapy and a potential therapeutic target in overcoming resistance to treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Yang M, Zhao J, Liu T, Yang X, Wei H, Xu W, et al. Use of FLT3 inhibitors in acute myeloid leukemia remission induction or salvage therapy: systematic review and meta-analysis. Cancer Manag Res. 2018;10:2635–52.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–98.
Zhou J, Chng WJ. Resistance to FLT3 inhibitors in acute myeloid leukemia: molecular mechanisms and resensitizing strategies. World J Clin Oncol. 2018;9:90–7.
Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018;17:57–78.
Basu B, Saha G, Choudhury SG, Ghosh MK. Cellular homeostasis or tumorigenesis: USP7 playing the double agent. Cancer Cell Microenviron. 2017;4:e1624.
Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–86.
Bhattacharya S, Chakraborty D, Basu M, Ghosh MK. Emerging insights into HAUSP (USP7) in physiology, cancer and other diseases. Signal Transduct Target Ther. 2018;3:17.
Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455:813–7.
van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–73.
Morotti A, Panuzzo C, Crivellaro S, Pergolizzi B, Familiari U, Berger AH, et al. BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia. 2014;28:1326–33.
D’Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharm Ther. 2015;147:32–54.
Kategaya L, Di Lello P, Rougé L, Pastor R, Clark KR, Drummond J, et al. USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature. 2017;550:534–8.
Turnbull AP, Ioannidis S, Krajewski WW, Pinto-Fernandez A, Heride C, Martin ACL, et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature. 2017;550:481–6.
Lamberto I, Liu X, Seo HS, Schauer NJ, Iacob RE, Hu W, et al. Structure-guided development of a potent and selective non-covalent active-site inhibitor of USP7. Cell Chem Biol. 2017;24:1490–500.
Pozhidaeva A, Valles G, Wang F, Wu J, Sterner DE, Nguyen P, et al. USP7-specific inhibitors target and modify the enzyme’s active site via distinct chemical mechanisms. Cell Chem Biol. 2017;24:1501–12.
Desroses M, Altun M. The next step forward in ubiquitin-specific protease 7 selective inhibition. Cell Chem Biol. 2017;24:1429–31.
Gavory G, O’Dowd CR, Helm MD, Flasz J, Arkoudis E, Dossang A, et al. Discovery and characterization of highly potent and selective allosteric USP7 inhibitors. Nat Chem Biol. 2018;14:118–25.
Carra G, Panuzzo C, Torti D, Parvis G, Crivellaro S, Familiari U, et al. Therapeutic Inhibition of USP7-PTEN network in chronic lymphocytic leukemia: a strategy to overcoming TP53 mutated/deleted clones. Oncotarget. 2017;8:35508–22.
Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–58.
Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 2013;4:e867.
Adam K, Cartel M, Lambert M, David L, Yuan L, Besson A et al. A PIM-CHK1 signaling pathway regulates PLK1 phosphorylation and function during mitosis. J Cell Sci. 2018;10:131.
David L, Fernandez-Vidal A, Bertoli S, Grgurevic S, Lepage B, Deshaies D, et al. CHK1 as a therapeutic target to bypass chemoresistance in AML. Sci Signal. 2016;9:ra90.
Network TCGAR. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Verhaak RG, Wouters BJ, Erpelinck CA, Abbas S, Beverloo HB, Lugthart S, et al. Prediction of molecular subtypes in acute myeloid leukemia based on gene expression profiling. Haematologica. 2009;94:131–4.
Hackl H, Steinleitner K, Lind K, Hofer S, Tosic N, Pavlovic S, et al. A gene expression profile associated with relapse of cytogenetically normal acute myeloid leukemia is enriched for leukemia stem cell genes. Leuk Lymphoma. 2015;56:1126–8.
Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, et al. Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discov. 2017;7:716–35.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–93.
Alonsa de Vega I, Martin Y, Smits VAJ. USP7 controls Chk1 protein stability by direct deubiquitination. Cell Cycle. 2014;13:3921–6.
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y, Zhang J, et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat Cell Biol. 2014;16:864–75.
An T, Gong Y, Li X, Kong L, Ma P, Gong L, et al. USP7 inhibitor P5091 inhibits wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131:29–39.
Petermann E, Maya-Mendoza A, Zachos G, Gillespie DA, Jackson DA, Caldecott KW. CHK1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol. 2006;26:3319–26.
Lecona E, Rodriguez-Acebes S, Specks J, Lopez-Contreras AJ, Ruppen I, Murga M, et al. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat Struct Mol Biol. 2016;23:270–7.
Zhao GY, Lin ZW, Lu CL, Gu J, Yuan YF, Xu FK, et al. USP7 overexpression predicts poor prognosis in lung squamous cell carcinoma and large cell carcinoma. Tumor Biol. 2015;36:1721–9.
Ma M, Yu N. Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. Onco Targets Ther. 2016;9:1559–69.
Shan H, Li X, Xiao X, Dai Y, Huang J, Song J, et al. USP7 deubiquitinates and stabilizes NOTCH1 in T-cell acute lymphoblastic leukemia. Signal Transduct Target Ther. 2018;3:29.
Jin Q, Martinez CA, Arcipowski KM, Zhu Y, Gutierrez-Diaz BT, Wang KK, et al. USP7 cooperates with NOTCH1 to drive the oncogenic transcriptional program in T cell leukemia. Clin Cancer Res. 2018;25:222–39.
Lobry C, Oh P, Mansour MR, Look AT, Aifantis I. Notch signaling: switching an oncogene to a tumor suppressor. Blood. 2014;123:2451–9.
Wang S, Itoh M, Shiratori E, Ohtaka M, Tohda S. NOTCH activation promotes glycosyltransferase expression in human myeloid leukemia cells. Hematol Rep. 2018;10:7576.
Xu X, Zhao Y, Xu M, Dai Q, Meng W, Yang J, et al. Activation of notch signal pathway is associated with a poorer prognosis in acute myeloid leukemia. Med Oncol. 2011;28:483–9.
Takam Kamga P, Bassi G, Cassaro A, Midolo M, Di Trapani M, Gatti A, et al. Notch signaling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget. 2016;7:21713–27.
Acknowledgements
We would like to sincerely thank Thomas Farge for his technical support during in vivo experiments and Alison Boutet for technical assistance. We thank Laetitia Ligat and Dr Marie Tosolini at the CRCT microscopy facility and Manon Farcé at the CRCT cytometry facility for their helpful discussions of the analyses and the development of macro used in the ImageJ software. We gratefully acknowledge Dr Ze’ev Ronai for the critical review of the manuscript, and sincerely thank all members of the “Cell Cycle and Cancer” team for their critical review during the entire study. The authors sincerely thank Chloé Laplagne for the technical PBMC preparation and Dr Marie Jeanne Pillaire for insightful discussions on DNA fiber spreading experiments. Maëlle Cartel is a recipient of a fellowship from the Ligue Nationale contre le Cancer. We are grateful to our healthcare professionals for their boundless investment during the COVID-19 crisis.
Author information
Authors and Affiliations
Contributions
MC, PLM, MG, LD, JES, SM, and CD conceived and designed the study; MC, PLM, MG, LD, and CD performed experiments; SB and VMDM. contributed clinical samples; MC, AB, JES, SM, and CD wrote the manuscript; AB, JES, SM, and CD insured administrative, technical and material support; SM and CD supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cartel, M., Mouchel, PL., Gotanègre, M. et al. Inhibition of ubiquitin-specific protease 7 sensitizes acute myeloid leukemia to chemotherapy. Leukemia 35, 417–432 (2021). https://doi.org/10.1038/s41375-020-0878-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-020-0878-x
This article is cited by
-
Role of USP7 in the regulation of tolerogenic dendritic cell function in type 1 diabetes
Cellular & Molecular Biology Letters (2025)
-
Ubiquitin-specific proteases (USPs) in leukemia: a systematic review
BMC Cancer (2024)
-
USP47 stabilizes YBX1 to promote the progression of acute myeloid leukemia
Oncogene (2024)
-
The roles of ubiquitination in AML
Annals of Hematology (2024)
-
Protein degradation: expanding the toolbox to restrain cancer drug resistance
Journal of Hematology & Oncology (2023)